Features of cardiomyocyte proliferation and its potential for cardiac regeneration
- PMID: 18662194
- PMCID: PMC4514102
- DOI: 10.1111/j.1582-4934.2008.00439.x
Features of cardiomyocyte proliferation and its potential for cardiac regeneration
Abstract
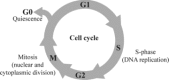
The human heart does not regenerate. Instead, following injury, human hearts scar. The loss of contractile tissue contributes significantly to morbidity and mortality. In contrast to humans, zebrafish and newts faithfully regenerate their hearts. Interestingly, regeneration is in both cases based on cardiomyocyte proliferation. In addition, mammalian cardiomyocytes proliferate during foetal development. Their proliferation reaches its maximum around chamber formation, stops shortly after birth, and subsequent heart growth is mostly achieved by an increase in cardiomyocyte size (hypertrophy). The underlying mechanisms that regulate cell cycle arrest and the switch from proliferation to hypertrophy are unclear. In this review, we highlight features of dividing cardiomyocytes, summarize the attempts to induce mammalian cardiomyocyte proliferation, critically discuss methods commonly used for its detection, and explore the potential and problems of inducing cardiomyocyte proliferation to improve function in diseased hearts.
Figures


References
-
- Rosamond W, Flegal K, Friday G, Furie K, Go A, Greenlund K, Haase N, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O'Donnell CJ, Roger V, Rumsfeld J, Sorlle P, Stelnberger J, Thorn T. Wasserthlel-Smoller S. Hong Y. Heart disease and stroke statistics–2007 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2007;115:e69–171. - PubMed
-
- Mummery CL. Cardiology: solace for the broken-hearted? Nature. 2005;433:585–7. - PubMed
-
- Rumyantsev PP. Interrelations of the proliferation and differentiation processes during cardiact myogenesis and regeneration. Int Rev Cytol. 1977;51:186–273. - PubMed
-
- Pasumarthi KB, Field LJ. Cardiomyocyte cell cycle regulation. Circ Res. 2002;90:1044–54. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

