Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects
- PMID: 18662287
- PMCID: PMC2526242
- DOI: 10.1111/j.1365-2125.2008.03250.x
Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects
Abstract
Aim: To characterize the effects of lamotrigine on QT interval in healthy subjects.
Methods: Healthy subjects received a single oral dose of moxifloxacin (400 mg) or placebo in crossover design, followed by a dose-escalating regimen of lamotrigine (n = 76) over a 77-day period, or matched placebo (n = 76). Blood samples were taken for determination of moxifloxacin and lamotrigine concentrations and digital 12-lead ECGs were recorded. The relationships between individual QT values and respective individual moxifloxacin or lamotrigine concentrations were explored using population pharmacokinetic-pharmacodynamic (PK-PD) modelling.
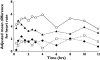
Results: Moxifloxacin was associated with a maximum mean increase from baseline in QTcF of 14.81 ms [90% confidence interval (CI) 13.50, 16.11] 2.5 h after dosing. Steady-state exposure to lamotrigine (50, 150 or 200 mg b.d.) was not associated with an increase in QTc interval. Small reductions in QTcF (maximum mean difference from placebo -7.48 ms, 90% CI -10.49, -4.46) and small increases in heart rate (maximum mean difference from placebo 5.94 bpm, 90% CI 3.81, 8.06) were observed with lamotrigine 200 mg b.d. vs. placebo. No effect of lamotrigine on QRS duration or blood pressure was observed. No outliers with QTcF > 450 ms, or with an increase from baseline of >60 ms were observed in the lamotrigine group. PK-PD modelling indicated statistically significant decreases in individually corrected QT intervals for lamotrigine and statistically significant increases in individually corrected QT intervals for moxifloxacin over the concentration ranges studied.
Conclusions: Therapeutic doses of lamotrigine (50-200 mg b.d.) were not associated with QT prolongation in healthy subjects.
Figures




Comment in
-
Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects, Dixon et al. 2008; request for publication of PR interval data.Br J Clin Pharmacol. 2011 Jun;71(6):963. doi: 10.1111/j.1365-2125.2010.03856.x. Br J Clin Pharmacol. 2011. PMID: 21564166 Free PMC article. No abstract available.
Similar articles
-
Effect of single doses of maraviroc on the QT/QTc interval in healthy subjects.Br J Clin Pharmacol. 2008 Apr;65 Suppl 1(Suppl 1):68-75. doi: 10.1111/j.1365-2125.2008.03138.x. Br J Clin Pharmacol. 2008. PMID: 18333868 Free PMC article. Clinical Trial.
-
Ipragliflozin does not prolong QTc interval in healthy male and female subjects: a phase I study.Clin Ther. 2013 Aug;35(8):1150-1161.e3. doi: 10.1016/j.clinthera.2013.06.009. Epub 2013 Aug 2. Clin Ther. 2013. PMID: 23910665 Clinical Trial.
-
No effect of a single supratherapeutic dose of lersivirine, a next-generation nonnucleoside reverse transcriptase inhibitor, on corrected QT interval in healthy subjects.Antimicrob Agents Chemother. 2012 May;56(5):2408-13. doi: 10.1128/AAC.05194-11. Epub 2012 Feb 27. Antimicrob Agents Chemother. 2012. PMID: 22371898 Free PMC article. Clinical Trial.
-
Thorough QT Studies: Questions and Quandaries.Drug Saf. 2010 Jan 1;33(1):1-14. doi: 10.2165/11319160-000000000-00000. Drug Saf. 2010. PMID: 20000862 Review.
-
Early QT assessment--how can our confidence in the data be improved?Br J Clin Pharmacol. 2013 Nov;76(5):642-8. doi: 10.1111/bcp.12068. Br J Clin Pharmacol. 2013. PMID: 23278510 Free PMC article. Review.
Cited by
-
Effects of Subcutaneous Pasireotide on Cardiac Repolarization in Healthy Volunteers: a Single‐Center, Phase I, Randomized, Four‐Way Crossover Study.J Clin Pharmacol. 2014 Jan;54(1):75-86. doi: 10.1002/jcph.213. J Clin Pharmacol. 2014. PMID: 24242903 Free PMC article. Clinical Trial.
-
Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects, Dixon et al. 2008; request for publication of PR interval data.Br J Clin Pharmacol. 2011 Jun;71(6):963. doi: 10.1111/j.1365-2125.2010.03856.x. Br J Clin Pharmacol. 2011. PMID: 21564166 Free PMC article. No abstract available.
-
Orally administered moxifloxacin prolongs QTc in healthy Chinese volunteers: a randomized, single-blind, crossover study.Acta Pharmacol Sin. 2015 Apr;36(4):448-53. doi: 10.1038/aps.2014.153. Epub 2015 Mar 23. Acta Pharmacol Sin. 2015. PMID: 25832425 Free PMC article. Clinical Trial.
-
Ventricular tachycardia associated with lacosamide co-medication in drug-resistant epilepsy.Epilepsy Behav Case Rep. 2012 Nov 7;1:26-8. doi: 10.1016/j.ebcr.2012.10.001. eCollection 2013. Epilepsy Behav Case Rep. 2012. PMID: 25688050 Free PMC article.
-
Update on the evaluation of a new drug for effects on cardiac repolarization in humans: issues in early drug development.Br J Pharmacol. 2010 Jan;159(1):34-48. doi: 10.1111/j.1476-5381.2009.00427.x. Epub 2009 Sep 23. Br J Pharmacol. 2010. PMID: 19775279 Free PMC article. Review.
References
-
- Hoffmann P, Warner B. Are hERG channel inhibition and QT interval prolongation all there is in drug-induced torsadogenesis? A review of emerging trends. J Pharmacol Toxicol Methods. 2006;53:87–105. - PubMed
-
- Danielsson BR, Lansdell K, Patmore L, Tomson T. Effects of antiepileptic drugs lamotrigine, topiramate and gabapentin on hERG potassium currents. Epilepsy Res. 2005;63:17–25. - PubMed
-
- Danielsson BR, Lansdell K, Patmore L, Tomson T. Phenytoin and phenobarbital inhibit human hERG potassium channels. Epilepsy Res. 2003;55:147–57. - PubMed
-
- Zünkler BJ. Human ether-a-go-go-related (HERG) gene and ATP-sensitive potassium channels as targets for adverse drug effects. Pharmacol Ther. 2006;112:12–37. - PubMed
-
- Johannessen SI, Battino D, Berry DJ, Bialer M, Krämer G, Tomson T, Patsalos PN. Therapeutic drug monitoring of the newer antiepileptic drugs. Ther Drug Monit. 2003;25:347–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

