Mouse early oocytes are transiently polar: three-dimensional and ultrastructural analysis
- PMID: 18662685
- PMCID: PMC2613006
- DOI: 10.1016/j.yexcr.2008.07.007
Mouse early oocytes are transiently polar: three-dimensional and ultrastructural analysis
Abstract
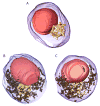
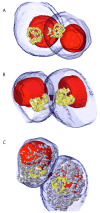
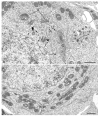
The oocytes of many invertebrate and non-mammalian vertebrate species are not only asymmetrical but also polar in the distribution of organelles, localized RNAs and proteins, and the oocyte polarity dictates the patterning of the future embryo. Polarily located within the oocytes of many species is the Balbiani body (Bb), which in Xenopus is known to be associated with the germinal granules responsible for the determination of germ cell fate. In contrast, in mammals, it is widely believed that the patterning of the embryo does not occur before implantation, and that oocytes are non-polar and symmetrical. Although the oocytes of many mammals, including mice and humans, contain Bbs, it remains unknown how and if the presence of Bbs relates to mouse oocyte and egg polarity. Using three-dimensional reconstruction of mouse neonatal oocytes, we showed that mouse early oocytes are both asymmetrical and transiently polar. In addition, the specifics of polarity in mouse oocytes are highly reminiscent of those in Xenopus early oocytes. Based on these findings, we conclude that the polarity of early oocytes imposed by the position of the centrioles at the cytoplasmic bridges is a fundamental and ancestral feature across the animal kingdom.
Figures




Similar articles
-
Balbiani cytoplasm in oocytes of a primitive fish, the sturgeon Acipenser gueldenstaedtii, and its potential homology to the Balbiani body (mitochondrial cloud) of Xenopus laevis oocytes.Cell Tissue Res. 2007 Jul;329(1):137-45. doi: 10.1007/s00441-007-0403-9. Epub 2007 Mar 16. Cell Tissue Res. 2007. PMID: 17364198
-
Methods for the analysis of early oogenesis in Zebrafish.Dev Biol. 2017 Oct 15;430(2):310-324. doi: 10.1016/j.ydbio.2016.12.014. Epub 2016 Dec 14. Dev Biol. 2017. PMID: 27988227 Free PMC article.
-
Bucky ball functions in Balbiani body assembly and animal-vegetal polarity in the oocyte and follicle cell layer in zebrafish.Dev Biol. 2008 Sep 1;321(1):40-50. doi: 10.1016/j.ydbio.2008.05.557. Epub 2008 Jun 9. Dev Biol. 2008. PMID: 18582455 Free PMC article.
-
The vertebrate Balbiani body, germ plasm, and oocyte polarity.Curr Top Dev Biol. 2019;135:1-34. doi: 10.1016/bs.ctdb.2019.04.003. Epub 2019 May 3. Curr Top Dev Biol. 2019. PMID: 31155356 Review.
-
RNA localization and germ cell determination in Xenopus.Int Rev Cytol. 2001;203:63-91. doi: 10.1016/s0074-7696(01)03004-2. Int Rev Cytol. 2001. PMID: 11131528 Review.
Cited by
-
Female Germline Cysts in Animals: Evolution and Function.Results Probl Cell Differ. 2024;71:23-46. doi: 10.1007/978-3-031-37936-9_2. Results Probl Cell Differ. 2024. PMID: 37996671 Review.
-
Premature ovarian failure in nobox-deficient mice is caused by defects in somatic cell invasion and germ cell cyst breakdown.J Assist Reprod Genet. 2011 Jul;28(7):583-9. doi: 10.1007/s10815-011-9553-5. Epub 2011 Mar 3. J Assist Reprod Genet. 2011. PMID: 21369782 Free PMC article.
-
Dynamic maternal synthesis and segregation of the germ plasm organizer, Bucky ball, in chicken oocytes and follicles.Sci Rep. 2024 Nov 12;14(1):27753. doi: 10.1038/s41598-024-78544-7. Sci Rep. 2024. PMID: 39532932 Free PMC article.
-
Cyclin B1 mRNA translation is temporally controlled through formation and disassembly of RNA granules.J Cell Biol. 2013 Sep 30;202(7):1041-55. doi: 10.1083/jcb.201302139. Epub 2013 Sep 23. J Cell Biol. 2013. PMID: 24062337 Free PMC article.
-
New Insights into the Mammalian Egg Zona Pellucida.Int J Mol Sci. 2021 Mar 23;22(6):3276. doi: 10.3390/ijms22063276. Int J Mol Sci. 2021. PMID: 33806989 Free PMC article. Review.
References
-
- Gurdon JB. The generation of diversity and pattern in animal development. Cell. 1992;68:185–199. - PubMed
-
- De Smedt V, Szollosi D, Kloc M. The Balbiani body: Asymmetry in the mammalian oocyte. Genesis. 2000;26:208–212. - PubMed
-
- Kloc M, Bilinski S, Etkin LD. The Balbiani body and germ cell determinants: 150 years later. Curr Topics Dev Biol. 2004b;59:1–36. - PubMed
-
- Wodarz A. Establishing cell polarity in development. Nat Cell Biol. 2002;4:E39–44. - PubMed
-
- Heasman J. Patterning the early Xenopus embryo. Development. 2006a;133:1205–17. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

