Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen
- PMID: 18662693
- PMCID: PMC3537501
- DOI: 10.1016/j.jim.2008.07.005
Optimization of methods to study pulmonary dendritic cell migration reveals distinct capacities of DC subsets to acquire soluble versus particulate antigen
Abstract
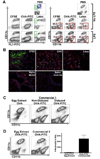
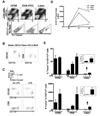
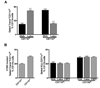
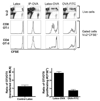
Dendritic cell migration from the airway to lymph nodes is a key event in the development of airway immunity during infection, allergy, and vaccination. To identify the best approaches to investigate DC migration to lung-draining lymph nodes, we directly compared three methods previously used to track DC migration: airway administration of fluorescent OVA, latex beads, or carboxyfluorescein succinimidyl ester (CFSE). We show that two of the methods employed in optimal conditions-administration of fluorescent OVA or latex particles-have broadly relevant utility in studies of pulmonary DC migration, both in the presence and absence of inflammatory mediators. However, CFSE was of limited value because it induced a robust airway inflammatory response upon instillation. Unexpectedly, antigen-loaded tracers with distinct physical properties differently affected the populations that acquired the tracers and the overall T cell response. Specifically, soluble OVA and OVA formulated as a particulate after conjugation to latex beads were acquired in different proportions in vivo by the two characterized subsets of pulmonary DCs: CD11b(hi)CD103(-) and CD11b(lo)CD103(+)langerin(+) DC populations. Consequently, and in line with recent studies that these two subsets of DCs respectively activate CD4(+) and CD8(+) lymphocyte populations, the physical nature of the antigen delivery vehicle strongly influenced the degree of CD4(+) versus CD8(+) OVA-specific T cell activation. This finding suggests that changes in the physical presentation of the same antigen delivered to the airway during natural immune responses or vaccinations may markedly affect the character of the T cell response that ensues.
Figures


References
-
- Barnden MJ, Allison J, Heath WR, Carbone FR. Defective TCR expression in transgenic mice constructed using cDNA-based alpha- and beta-chain genes under the control of heterologous regulatory elements. Immunol Cell Biol. 1998;76:34–40. - PubMed
-
- Beaty SR, Rose CE, Jr, Sung SS. Diverse and Potent Chemokine Production by Lung CD11bhigh Dendritic Cells in Homeostasis and in Allergic Lung Inflammation. J Immunol. 2007;178:1882–1895. - PubMed
-
- Belz GT, Bedoui S, Kupresanin F, Carbone FR, Heath WR. Minimal activation of memory CD8+ T cell by tissue-derived dendritic cells favors the stimulation of naive CD8+ T cells. Nat Immunol. 2007;8:1060–1066. - PubMed
-
- Belz GT, Smith CM, Kleinert L, Reading P, Brooks A, Shortman K, Carbone FR, Heath WR. Distinct migrating and nonmigrating dendritic cell populations are involved in MHC class I-restricted antigen presentation after lung infection with virus. Proc Natl Acad Sci U S A. 2004;101:8670–8675. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

