Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat
- PMID: 18662986
- PMCID: PMC2562054
- DOI: 10.1074/jbc.M804393200
Glyceroneogenesis is the dominant pathway for triglyceride glycerol synthesis in vivo in the rat
Abstract
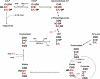
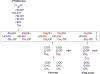
Triglyceride synthesis in mammalian tissues requires glycerol 3-phosphate as the source of triglyceride glycerol. In this study the relative contribution of glyceroneogenesis and glycolysis to triglyceride glycerol synthesis was quantified in vivo in adipose tissue, skeletal muscle, and liver of the rat in response to a chow diet (controls), 48-h fast, and lipogenic (high sucrose) diet. The rate of glyceroneogenesis was quantified using the tritium ([(3)H(2)]O) labeling of body water, and the contribution of glucose, via glycolysis, was determined using a [U-(14)C]glucose tracer. In epididymal and mesenteric adipose tissue of control rats, glyceroneogenesis accounted for approximately 90% of triglyceride glycerol synthesis. Fasting for 48 h did not alter glyceroneogenesis in adipose tissue, whereas the contribution of glucose was negligible. In response to sucrose feeding, the synthesis of triglyceride glycerol via both glyceroneogenesis and glycolysis nearly doubled (versus controls); however, glyceroneogenesis remained quantitatively higher as compared with the contribution of glucose. Enhancement of triglyceride-fatty acid cycling by epinephrine infusion resulted in a higher rate of glyceroneogenesis in adipose tissue, as compared with controls, whereas the contribution of glucose via glycolysis was not measurable. Glyceroneogenesis provided the majority of triglyceride glycerol in the gastrocnemius and soleus. In the liver the fractional contribution of glyceroneogenesis remained constant (approximately 60%) under all conditions and was higher than that of glucose. Thus, glyceroneogenesis, in contrast to glucose, via glycolysis, is quantitatively the predominant source of triglyceride glycerol in adipose tissue, skeletal muscle, and liver of the rat during fasting and high sucrose feeding.
Figures



References
-
- Newsholme, E. A., and Crabtree, B. (1976) Biochem. Soc. Symp. 61-109 - PubMed
-
- Reshef, L., Olswang, Y., Cassuto, H., Blum, B., Croniger, C. M., Kalhan, S. C., Tilghman, S. M., and Hanson, R. W. (2003) J. Biol. Chem. 278 30413-30416 - PubMed
-
- Robinson, J., and Newsholme, E. A. (1967) Biochem. J. 104 2-4
-
- Ballard, F. J., Hanson, R. W., and Leveille, G. A. (1967) J. Biol. Chem. 242 2746-2750 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

