Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension
- PMID: 18663089
- PMCID: PMC3920834
- DOI: 10.1161/CIRCULATIONAHA.107.736801
Genetic ablation of the BMPR2 gene in pulmonary endothelium is sufficient to predispose to pulmonary arterial hypertension
Abstract
Background: Pulmonary arterial hypertension (PAH) is a rare but fatal lung disease of diverse origins. PAH is now further subclassified as idiopathic PAH, familial PAH, and associated PAH varieties. Heterozygous mutations in BMPR2 can be detected in 50% to 70% of patients with familial PAH and 10% to 40% of patients with idiopathic PAH. Although endothelial cells have been suspected as the cellular origin of PAH pathogenesis, no direct in vivo evidence has been clearly presented. The present study was designed to investigate whether endothelial Bmpr2 deletion can predispose to PAH.
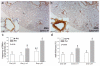
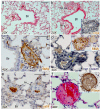
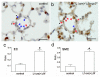
Methods and results: The Bmpr2 gene was deleted in pulmonary endothelial cells using Bmpr2 conditional knockout mice and a novel endothelial Cre transgenic mouse line. Wide ranges of right ventricular systolic pressure were observed in mice with heterozygous (21.7 to 44.1 mm Hg; median, 23.7 mm Hg) and homozygous (20.7 to 56.3 mm Hg; median, 27 mm Hg) conditional deletion of Bmpr2 in pulmonary endothelial cells compared with control mice (19.9 to 26.7 mm Hg; median, 23 mm Hg) at 2 to 7 months of age. A subset of mice with right ventricular systolic pressure >30 mm Hg exhibited right ventricular hypertrophy and an increase in the number and wall thickness of muscularized distal pulmonary arteries. In the lungs of these mice with high right ventricular systolic pressure, the expression of proteins involved in the pathogenesis of PAH such as serotonin transporter and tenascin-C was elevated in distal arteries and had a high incidence of perivascular leukocyte infiltration and in situ thrombosis.
Conclusions: Conditional heterozygous or homozygous Bmpr2 deletion in pulmonary endothelial cells predisposes mice to develop PAH.
Figures


Similar articles
-
Autophagy contributes to BMP type 2 receptor degradation and development of pulmonary arterial hypertension.J Pathol. 2019 Nov;249(3):356-367. doi: 10.1002/path.5322. Epub 2019 Aug 27. J Pathol. 2019. PMID: 31257577 Free PMC article.
-
Inflammation, endothelial injury, and persistent pulmonary hypertension in heterozygous BMPR2-mutant mice.Am J Physiol Heart Circ Physiol. 2008 Aug;295(2):H677-90. doi: 10.1152/ajpheart.91519.2007. Epub 2008 Jun 13. Am J Physiol Heart Circ Physiol. 2008. PMID: 18552156 Free PMC article.
-
Physiologic and molecular consequences of endothelial Bmpr2 mutation.Respir Res. 2011 Jun 22;12(1):84. doi: 10.1186/1465-9921-12-84. Respir Res. 2011. PMID: 21696628 Free PMC article.
-
BMP type II receptor as a therapeutic target in pulmonary arterial hypertension.Cell Mol Life Sci. 2017 Aug;74(16):2979-2995. doi: 10.1007/s00018-017-2510-4. Epub 2017 Apr 26. Cell Mol Life Sci. 2017. PMID: 28447104 Free PMC article. Review.
-
The Role of Bone Morphogenetic Protein Receptor Type 2 (BMPR2) and the Prospects of Utilizing Induced Pluripotent Stem Cells (iPSCs) in Pulmonary Arterial Hypertension Disease Modeling.Cells. 2022 Nov 29;11(23):3823. doi: 10.3390/cells11233823. Cells. 2022. PMID: 36497082 Free PMC article. Review.
Cited by
-
Pulmonary artery smooth muscle hypertrophy: roles of glycogen synthase kinase-3beta and p70 ribosomal S6 kinase.Am J Physiol Lung Cell Mol Physiol. 2010 Jun;298(6):L793-803. doi: 10.1152/ajplung.00108.2009. Epub 2010 Feb 26. Am J Physiol Lung Cell Mol Physiol. 2010. PMID: 20190034 Free PMC article.
-
The Latest in Animal Models of Pulmonary Hypertension and Right Ventricular Failure.Circ Res. 2022 Apr 29;130(9):1466-1486. doi: 10.1161/CIRCRESAHA.121.319971. Epub 2022 Apr 28. Circ Res. 2022. PMID: 35482834 Free PMC article. Review.
-
Bone Morphogenetic Proteins in Vascular Homeostasis and Disease.Cold Spring Harb Perspect Biol. 2018 Feb 1;10(2):a031989. doi: 10.1101/cshperspect.a031989. Cold Spring Harb Perspect Biol. 2018. PMID: 28348038 Free PMC article. Review.
-
Implication of overexpression of dishevelled-associated activator of morphogenesis 1 (Daam-1) for the pathogenesis of human Idiopathic Pulmonary Arterial Hypertension (IPAH).Diagn Pathol. 2017 Mar 14;12(1):25. doi: 10.1186/s13000-017-0614-7. Diagn Pathol. 2017. PMID: 28288669 Free PMC article.
-
Endothelial Dysfunction in Pulmonary Hypertension: Cause or Consequence?Biomedicines. 2021 Jan 9;9(1):57. doi: 10.3390/biomedicines9010057. Biomedicines. 2021. PMID: 33435311 Free PMC article. Review.
References
-
- Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, Gibbs S, Lebrec D, Speich R, Beghetti M, Rich S, Fishman A. Clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2004;43:5S–12S. - PubMed
-
- Humbert M, Morrell NW, Archer SL, Stenmark KR, MacLean MR, Lang IM, Christman BW, Weir EK, Eickelberg O, Voelkel NF, Rabinovitch M. Cellular and molecular pathobiology of pulmonary arterial hypertension. J Am Coll Cardiol. 2004;43:13S–24S. - PubMed
-
- McLaughlin VV, Presberg KW, Doyle RL, Abman SH, McCrory DC, Fortin T, Ahearn G. Prognosis of pulmonary arterial hypertension: ACCP evidence-based clinical practice guidelines. Chest. 2004;126:78S–92S. - PubMed
-
- McLaughlin VV, McGoon MD. Pulmonary arterial hypertension. Circulation. 2006;114:1417–1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

