Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor
- PMID: 18663128
- PMCID: PMC2525581
- DOI: 10.1084/jem.20080397
Human interleukin 17-producing cells originate from a CD161+CD4+ T cell precursor
Abstract
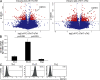
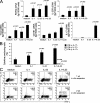
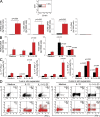
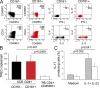
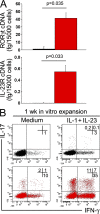
We demonstrate that CD161 is a highly up-regulated gene in human interleukin (IL) 17 T helper cell (Th17) clones and that all IL-17-producing cells are contained in the CD161(+) fraction of CD4(+) T cells present in the circulation or in inflamed tissues, although they are not CD1-restricted natural killer T cells. More importantly, we show that all IL-17-producing cells originate from CD161(+) naive CD4(+) T cells of umbilical cord blood, as well as of the postnatal thymus, in response to the combined activity of IL-1 beta and IL-23. These findings implicate CD161 as a novel surface marker for human Th17 cells and demonstrate the exclusive origin of these cells from a CD161(+)CD4(+) T cell progenitor.
Figures



References
-
- Abbas, A.K., K.M. Murphy, and A. Sher. 1996. Functional diversity of helper T lymphocytes. Nature. 383:787–793. - PubMed
-
- Romagnani, S. 1997. The Th1/Th2 paradigm. Immunol. Today. 18:263–266. - PubMed
-
- Rengarajan, J., S.J. Szabo, and L.H. Glimcher. 2000. Transcriptional regulation of Th1/Th2 polarization. Immunol. Today. 21:479–483. - PubMed
-
- Aggarwal, S., N. Ghilardi, M.H. Xie, F.J. de Sauvage, and A.L. Gurney. 2003. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J. Biol. Chem. 278:1910–1914. - PubMed
-
- Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

