ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways
- PMID: 18663135
- PMCID: PMC2483331
- DOI: 10.1085/jgp.200810003
ACTH inhibits bTREK-1 K+ channels through multiple cAMP-dependent signaling pathways
Abstract
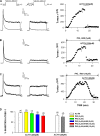
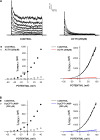
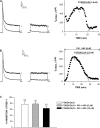
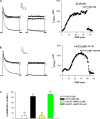
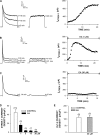
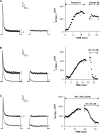
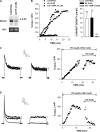
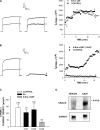
Bovine adrenal zona fasciculata (AZF) cells express bTREK-1 K(+) channels that set the resting membrane potential and function pivotally in the physiology of cortisol secretion. Inhibition of these K(+) channels by adrenocorticotropic hormone (ACTH) or cAMP is coupled to depolarization and Ca(2+) entry. The mechanism of ACTH and cAMP-mediated inhibition of bTREK-1 was explored in whole cell patch clamp recordings from AZF cells. Inhibition of bTREK-1 by ACTH and forskolin was not affected by the addition of both H-89 and PKI (6-22) amide to the pipette solution at concentrations that completely blocked activation of cAMP-dependent protein kinase (PKA) in these cells. The ACTH derivative, O-nitrophenyl, sulfenyl-adrenocorticotropin (NPS-ACTH), at concentrations that produced little or no activation of PKA, inhibited bTREK-1 by a Ca(2+)-independent mechanism. Northern blot analysis showed that bovine AZF cells robustly express mRNA for Epac2, a guanine nucleotide exchange protein activated by cAMP. The selective Epac activator, 8-pCPT-2'-O-Me-cAMP, applied intracellularly through the patch pipette, inhibited bTREK-1 (IC(50) = 0.63 microM) at concentrations that did not activate PKA. Inhibition by this agent was unaffected by PKA inhibitors, including RpcAMPS, but was eliminated in the absence of hydrolyzable ATP. Culturing AZF cells in the presence of ACTH markedly reduced the expression of Epac2 mRNA. 8-pCPT-2'-O-Me-cAMP failed to inhibit bTREK-1 current in AZF cells that had been treated with ACTH for 3-4 d while inhibition by 8-br-cAMP was not affected. 8-pCPT-2'-O-Me-cAMP failed to inhibit bTREK-1 expressed in HEK293 cells, which express little or no Epac2. These findings demonstrate that, in addition to the well-described PKA-dependent TREK-1 inhibition, ACTH, NPS-ACTH, forskolin, and 8-pCPT-2'-O-Me-cAMP also inhibit these K(+) channels by a PKA-independent signaling pathway. The convergent inhibition of bTREK-1 through parallel PKA- and Epac-dependent mechanisms may provide for failsafe membrane depolarization by ACTH.
Figures


References
-
- Awad, J.A., R.A. Johnson, K.H. Jakobs, and G. Schultz. 1983. Interactions of forskolin and adenylate cyclase. Effects on substrate kinetics and protection against inactivation by heat and N-ethylmaleimide. J. Biol. Chem. 258:2960–2965. - PubMed
-
- Bockenhauer, D., N. Zilberberg, and S.A. Goldstein. 2001. KCNK2: reversible conversion of a hippocampal potassium leak into a voltage-dependent channel. Nat. Neurosci. 4:486–491. - PubMed
-
- Bos, J.L. 2003. Epac: a new cAMP target and new avenues in cAMP research. Nat. Rev. Mol. Cell Biol. 4:733–738. - PubMed
-
- Brooks, S.P., and K.B. Storey. 1992. Bound and determined: a computer program for making buffers of defined ion concentrations. Anal. Biochem. 201:119–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

