Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers
- PMID: 18663144
- PMCID: PMC2483518
- DOI: 10.1083/jcb.200801027
Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers
Abstract
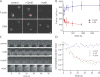
Turnover of actin filaments in cells requires rapid actin disassembly in a cytoplasmic environment that thermodynamically favors assembly because of high concentrations of polymerizable monomers. We here image the disassembly of single actin filaments by cofilin, coronin, and actin-interacting protein 1, a purified protein system that reconstitutes rapid, monomer-insensitive disassembly (Brieher, W.M., H.Y. Kueh, B.A. Ballif, and T.J. Mitchison. 2006. J. Cell Biol. 175:315-324). In this three-component system, filaments disassemble in abrupt bursts that initiate preferentially, but not exclusively, from both filament ends. Bursting disassembly generates unstable reaction intermediates with lowered affinity for CapZ at barbed ends. CapZ and cytochalasin D (CytoD), a barbed-end capping drug, strongly inhibit bursting disassembly. CytoD also inhibits actin disassembly in mammalian cells, whereas latrunculin B, a monomer sequestering drug, does not. We propose that bursts of disassembly arise from cooperative separation of the two filament strands near an end. The differential effects of drugs in cells argue for physiological relevance of this new disassembly pathway and potentially explain discordant results previously found with these drugs.
Figures


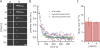




References
-
- Amberg, D.C., E. Basart, and D. Botstein. 1995. Defining protein interactions with yeast actin in vivo. Nat. Struct. Biol. 2:28–35. - PubMed
-
- Andrianantoandro, E., and T.D. Pollard. 2006. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell. 24:13–23. - PubMed
-
- Balcer, H.I., A.L. Goodman, A.A. Rodal, E. Smith, J. Kugler, J.E. Heuser, and B.L. Goode. 2003. Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13:2159–2169. - PubMed
-
- Blanchoin, L., and T.D. Pollard. 1999. Mechanism of interaction of Acanthamoeba actophorin (ADF/Cofilin) with actin filaments. J. Biol. Chem. 274:15538–15546. - PubMed
-
- Bobkov, A.A., A. Muhlrad, A. Shvetsov, S. Benchaar, D. Scoville, S.C. Almo, and E. Reisler. 2004. Cofilin (ADF) affects lateral contacts in F-actin. J. Mol. Biol. 337:93–104. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

