Biaryl phosphane ligands in palladium-catalyzed amination
- PMID: 18663711
- PMCID: PMC3517088
- DOI: 10.1002/anie.200800497
Biaryl phosphane ligands in palladium-catalyzed amination
Abstract
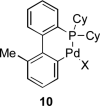
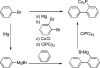
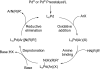
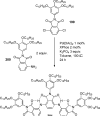
Palladium-catalyzed amination reactions of aryl halides have undergone rapid development in the last 12 years, largely driven by the implementation of new classes of ligands. Biaryl phosphanes have proven to provide especially active catalysts in this context. This Review discusses the application of these catalysts in C-N cross-coupling reactions in the synthesis of heterocycles and pharmaceuticals, in materials science, and in natural product synthesis.
Figures




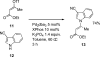











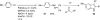

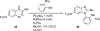
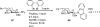
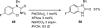

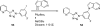


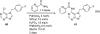






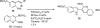



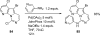

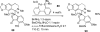








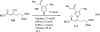
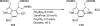
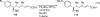


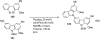





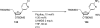
















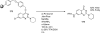
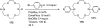





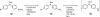
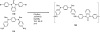





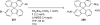
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

