Replication independent ATR signalling leads to G2/M arrest requiring Nbs1, 53BP1 and MDC1
- PMID: 18664457
- PMCID: PMC3708519
- DOI: 10.1093/hmg/ddn220
Replication independent ATR signalling leads to G2/M arrest requiring Nbs1, 53BP1 and MDC1
Abstract
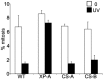
Ataxia telangiectasia and Rad3-related (ATR) is a phosphoinositol-3-kinase like kinase (PIKK) that initiates a signal transduction response to replication fork stalling. Defects in ATR signalling have been reported in several disorders characterized by microcephaly and growth delay. Here, we gain insight into factors influencing the ATR signalling pathway and consider how they can be exploited for diagnostic purposes. Activation of ATR at stalled replication forks leads to intra-S and G2/M phase checkpoint arrest. ATR also phosphorylates gamma-H2AX at single-stranded (ss) DNA regions generated during nucleotide excision repair (NER) in non-replicating cells, but the critical analysis of any functional consequence has not been reported. Here, we show that UV irradiation of G2 phase cells causes ATR-dependent but replication-independent G2/M checkpoint arrest. This process requires the Nbs1 N-terminus encompassing the FHA and BRCT domains but not the Nbs1 C-terminus in contrast to ATM-dependent activation of G2/M arrest in response to ionizing radiation. Thus, Nbs1 has a function in ATR signalling in a manner distinct to any role at stalled replication forks. Replication-independent ATR signalling also requires the mediator proteins, 53BP1 and MDC1, providing direct evidence for their role in ATR signalling, but not H2AX. Finally, the process is activated in Cockayne's syndrome but not Xeroderma pigmentosum group A cells providing evidence that ssDNA regions generated during NER are the ATR-pathway-specific activating lesion. Replication-independent G2/M checkpoint arrest represents a suitable assay to specifically identify patients with defective ATR signalling, including Seckel syndrome, Nijmegen breakage syndrome and MCPH-1-dependent primary microcephaly.
Figures
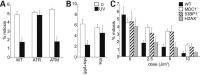
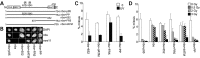

Similar articles
-
Nbs1 is required for ATR-dependent phosphorylation events.EMBO J. 2005 Jan 12;24(1):199-208. doi: 10.1038/sj.emboj.7600504. Epub 2004 Dec 16. EMBO J. 2005. PMID: 15616588 Free PMC article.
-
Role of ATM and the damage response mediator proteins 53BP1 and MDC1 in the maintenance of G(2)/M checkpoint arrest.Mol Cell Biol. 2010 Jul;30(13):3371-83. doi: 10.1128/MCB.01644-09. Epub 2010 Apr 26. Mol Cell Biol. 2010. PMID: 20421415 Free PMC article.
-
ATR-dependent phosphorylation and activation of ATM in response to UV treatment or replication fork stalling.EMBO J. 2006 Dec 13;25(24):5775-82. doi: 10.1038/sj.emboj.7601446. Epub 2006 Nov 23. EMBO J. 2006. PMID: 17124492 Free PMC article.
-
The role of NBS1 in the modulation of PIKK family proteins ATM and ATR in the cellular response to DNA damage.Cancer Lett. 2006 Nov 8;243(1):9-15. doi: 10.1016/j.canlet.2006.01.026. Epub 2006 Mar 10. Cancer Lett. 2006. PMID: 16530324 Free PMC article. Review.
-
The NBS1-ATM connection revisited.Cell Cycle. 2007 Oct 1;6(19):2366-70. doi: 10.4161/cc.6.19.4758. Epub 2007 Jul 18. Cell Cycle. 2007. PMID: 17881893 Review.
Cited by
-
Nucleotide excision repair-dependent DNA double-strand break formation and ATM signaling activation in mammalian quiescent cells.J Biol Chem. 2014 Oct 10;289(41):28730-7. doi: 10.1074/jbc.M114.589747. Epub 2014 Aug 27. J Biol Chem. 2014. PMID: 25164823 Free PMC article.
-
Role of RPA Phosphorylation in the ATR-Dependent G2 Cell Cycle Checkpoint.Genes (Basel). 2023 Dec 13;14(12):2205. doi: 10.3390/genes14122205. Genes (Basel). 2023. PMID: 38137027 Free PMC article.
-
Predictive factors of cytotoxic damage in radioactive iodine treatment of differentiated thyroid cancer patients.Mol Clin Oncol. 2015 May;3(3):692-698. doi: 10.3892/mco.2015.499. Epub 2015 Jan 27. Mol Clin Oncol. 2015. PMID: 26137289 Free PMC article.
-
The TRESLIN-MTBP complex couples completion of DNA replication with S/G2 transition.Mol Cell. 2022 Sep 15;82(18):3350-3365.e7. doi: 10.1016/j.molcel.2022.08.006. Epub 2022 Aug 31. Mol Cell. 2022. PMID: 36049481 Free PMC article.
-
ATM protein kinase: the linchpin of cellular defenses to stress.Cell Mol Life Sci. 2011 Sep;68(18):2977-3006. doi: 10.1007/s00018-011-0683-9. Epub 2011 May 2. Cell Mol Life Sci. 2011. PMID: 21533982 Free PMC article. Review.
References
-
- Kurz E.U., Lees-Miller S.P. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst) 2004;3:889–900. - PubMed
-
- Paull T.T., Rogakou E.P., Yamazaki V., Kirchgessner C.U., Gellert M., Bonner W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000;10:886–895. - PubMed
-
- Paulsen R.D., Cimprich K.A. The ATR pathway: fine-tuning the fork. DNA Repair (Amst) 2007;6:953–966. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

