Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain
- PMID: 18664652
- PMCID: PMC2575427
- DOI: 10.1093/jnci/djn216
Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain
Abstract
Background: The brain is increasingly being recognized as a sanctuary site for metastatic tumor cells in women with HER2-overexpressing breast cancer who receive trastuzumab therapy. There are no approved or widely accepted treatments for brain metastases other than steroids, cranial radiotherapy, and surgical resection. We examined the efficacy of lapatinib, an inhibitor of the epidermal growth factor receptor (EGFR) and HER2 kinases, for preventing the outgrowth of breast cancer cells in the brain in a mouse xenograft model of brain metastasis.
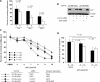
Methods: EGFR-overexpressing MDA-MB-231-BR (231-BR) brain-seeking breast cancer cells were transfected with an expression vector that contained or lacked the HER2 cDNA and used to examine the effect of lapatinib on the activation (ie, phosphorylation) of cell signaling proteins by immunoblotting, on cell growth by the tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assay, and on cell migration using a Boyden chamber assay. The outgrowth of large (ie, >50 microm(2)) and micrometastases was counted in brain sections from nude mice that had been injected into the left cardiac ventricle with 231-BR cells and, beginning 5 days later, treated by oral gavage with lapatinib or vehicle (n = 22-26 mice per treatment group). All statistical tests were two-sided.
Results: In vitro, lapatinib inhibited the phosphorylation of EGFR, HER2, and downstream signaling proteins; cell proliferation; and migration in 231-BR cells (both with and without HER2). Among mice injected with 231-BR-vector cells, those treated with 100 mg lapatinib/kg body weight had 54% fewer large metastases 24 days after starting treatment than those treated with vehicle (mean number of large metastases per brain section: 1.56 vs 3.36, difference = 1.80, 95% confidence interval [CI] = 0.92 to 2.68, P < .001), whereas treatment with 30 mg lapatinib/kg body weight had no effect. Among mice injected with 231-BR-HER2 cells, those treated with either dose of lapatinib had 50%-53% fewer large metastases than those treated with vehicle (mean number of large metastases per brain section, 30 mg/kg vs vehicle: 3.21 vs 6.83, difference = 3.62, 95% CI = 2.30 to 4.94, P < .001; 100 mg/kg vs vehicle: 3.44 vs 6.83, difference = 3.39, 95% CI = 2.08 to 4.70, P < .001). Immunohistochemical analysis revealed reduced phosphorylation of HER2 in 231-BR-HER2 cell-derived brain metastases from mice treated with the higher dose of lapatinib compared with 231-BR-HER2 cell-derived brain metastases from vehicle-treated mice (P < .001).
Conclusions: Lapatinib is the first HER2-directed drug to be validated in a preclinical model for activity against brain metastases of breast cancer.
Figures



References
-
- Lin N, Bellon J, Winer E. CNS metastases in breast cancer. J Clin Oncol. 2004;22(17):3608–3617. - PubMed
-
- Slotman B, Faive-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007;357(7):664–672. - PubMed
-
- Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549–558. - PubMed
-
- Patchell RA, Regine WF. The rationale for adjuvant whole brain radiation therapy with radiosurgery in the treatment of single brain metastases. Technol Cancer Res Treat. 2003;2(2):111–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

