A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer
- PMID: 18665164
- PMCID: PMC2527839
- DOI: 10.1038/sj.bjc.6604536
A randomised multicentre phase II trial of capecitabine vs S-1 as first-line treatment in elderly patients with metastatic or recurrent unresectable gastric cancer
Abstract
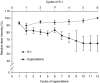
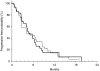
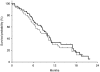
This randomised multicentre phase II study was conducted to investigate the activity and safety of two oral fluoropyrimidines, capecitabine or S-1, in elderly patients with advanced gastric cancer (AGC). Elderly (>or=65 years) chemo-naive patients with AGC were randomly assigned to receive capecitabine 1250 mg m(-2) two times daily on days 1-14 every 3 weeks or S-1 40-60 mg two times daily according to body surface area on days 1-28 every 6 weeks. Ninety-six patients were enrolled and 91 patients were randomised to capecitabine (N=46) or S-1 (N=45). Overall response rate, the primary end point, was 27.2% (95% CI, 14.1-40.4, 12 of 44 assessable patients) with capecitabine and 28.9% (95% CI, 15.6-42.1, 13 of 45) with S-1. Median times to progression and overall survival in the capecitabine arm (4.7 and 9.5 months, respectively) were similar to those in the S-1 arm (4.2 and 8.2 months, respectively). The incidence of grade 3-4 granulocytopenia was 6.8% with capecitabine and 4.8% with S-1. Grade 3-4 nonhaematologic toxicities were: asthenia (9.1% with capecitabine vs 7.1% with S-1), anorexia (6.8 vs 9.5%), diarrhoea (2.3 vs 0%), and hand-foot syndrome (6.8 vs 0%). Both capecitabine and S-1 monotherapies were active and tolerable as first-line treatment for elderly patients with AGC.
Figures
References
-
- Bajetta E, Procopio G, Celio L, Gattinoni L, Della Torre S, Mariani L, Catena L, Ricotta R, Longarini R, Zilembo N, Buzzoni R (2005) Safety and efficacy of two different doses of capecitabine in the treatment of advanced breast cancer in older women. J Clin Oncol 23: 2155–2161 - PubMed
-
- Blum JL, Jones SE, Buzdar AU, LoRusso PM, Kuter I, Vogel C, Osterwalder B, Burger HU, Brown CS, Griffin T (1999) Multicenter phase II study of capecitabine in paclitaxel-refractory metastatic breast cancer. J Clin Oncol 17: 485–493 - PubMed
-
- Cassidy J, Twelves C, Van Cutsem E, Hoff P, Bajetta E, Boyer M, Bugat R, Burger U, Garin A, Graeven U, McKendric J, Maroun J, Marshall J, Osterwalder B, Pérez-Manga G, Rosso R, Rougier P, Schilsky RL, Capecitabine Colorectal Cancer Study Group (2002) First-line oral capecitabine therapy in metastatic colorectal cancer: a favorable safety profile compared with intravenous 5-fluorouracil/leucovorin. Ann Oncol 13: 566–575 - PubMed
-
- Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40: 373–383 - PubMed
-
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR (2008) Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358: 36–46 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

