VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma
- PMID: 18665180
- PMCID: PMC2527820
- DOI: 10.1038/sj.bjc.6604508
VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma
Abstract
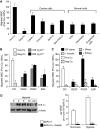
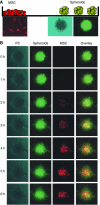
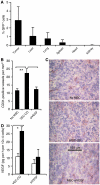
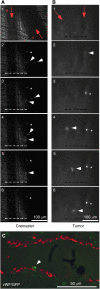
Little is known about the factors that enable the mobilisation of human mesenchymal stem cells (MSC) from the bone marrow into the blood stream and their recruitment to and retention in the tumour. We found specific migration of MSC towards growth factors present in pancreatic tumours, such as PDGF, EGF, VEGF and specific inhibitors Glivec, Erbitux and Avastin interfered with migration. Within a few hours, MSC migrated into spheroids consisting of pancreatic cancer cells, fibroblasts and endothelial cells as measured by time-lapse microscopy. Supernatant from subconfluent MSC increased sprouting of HUVEC due to VEGF production by MSC itself as demonstrated by RT-PCR and ELISA. Only few MSCs were differentiated into endothelial cells in vitro, whereas in vivo differentiation was not observed. Lentiviral GFP-marked MSCs, injected in nude mice xenografted with orthotopic pancreatic tumours, preferentially migrated into the tumours as observed by FACS analysis of green fluorescent cells. By immunofluorescence and intravital microscopic studies, we found the interaction of MSC with the endothelium of blood vessels. Mesenchymal stem cells supported tumour angiogenesis in vivo, that is CD31(+) vessel density was increased after the transfer of MSC compared with siVEGF-MSC. Our data demonstrate the migration of MSC toward tumour vessels and suggest a supportive role in angiogenesis.
Figures

Similar articles
-
Stroma-directed imatinib therapy impairs the tumor-promoting effect of bone marrow-derived mesenchymal stem cells in an orthotopic transplantation model of colon cancer.Int J Cancer. 2013 Feb 15;132(4):813-23. doi: 10.1002/ijc.27735. Epub 2012 Aug 6. Int J Cancer. 2013. PMID: 22821812
-
Down-regulation of platelet-derived growth factor-D inhibits cell growth and angiogenesis through inactivation of Notch-1 and nuclear factor-kappaB signaling.Cancer Res. 2007 Dec 1;67(23):11377-85. doi: 10.1158/0008-5472.CAN-07-2803. Cancer Res. 2007. Retraction in: Cancer Res. 2018 Sep 15;78(18):5469. doi: 10.1158/0008-5472.CAN-18-1173. PMID: 18056465 Retracted.
-
Inhibitory effect of bevacizumab on the angiogenesis and growth of retinoblastoma.Arch Ophthalmol. 2008 Jul;126(7):953-8. doi: 10.1001/archopht.126.7.953. Arch Ophthalmol. 2008. PMID: 18625942
-
Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors.Cancer Control. 2007 Jul;14(3):285-94. doi: 10.1177/107327480701400312. Cancer Control. 2007. PMID: 17615535 Review.
-
Mesenchymal stem cells and neovascularization: role of platelet-derived growth factor receptors.J Cell Mol Med. 2007 Sep-Oct;11(5):1012-30. doi: 10.1111/j.1582-4934.2007.00120.x. J Cell Mol Med. 2007. PMID: 17979880 Free PMC article. Review.
Cited by
-
The metastasis-promoting roles of tumor-associated immune cells.J Mol Med (Berl). 2013 Apr;91(4):411-29. doi: 10.1007/s00109-013-1021-5. Epub 2013 Mar 21. J Mol Med (Berl). 2013. PMID: 23515621 Free PMC article. Review.
-
Pharmacologically active microcarriers influence VEGF-A effects on mesenchymal stem cell survival.J Cell Mol Med. 2013 Jan;17(1):192-204. doi: 10.1111/j.1582-4934.2012.01662.x. Epub 2013 Jan 11. J Cell Mol Med. 2013. PMID: 23305078 Free PMC article.
-
Mesenchymal stem cells in preclinical cancer cytotherapy: a systematic review.Stem Cell Res Ther. 2018 Dec 7;9(1):336. doi: 10.1186/s13287-018-1078-8. Stem Cell Res Ther. 2018. PMID: 30526687 Free PMC article.
-
Mesenchymal Stromal Cell-Based Targeted Therapy Pancreatic Cancer: Progress and Challenges.Int J Mol Sci. 2023 Feb 10;24(4):3559. doi: 10.3390/ijms24043559. Int J Mol Sci. 2023. PMID: 36834969 Free PMC article. Review.
-
BMSC-HNC Interaction: Exploring Effects on Bone Integrity and Head and Neck Cancer Progression.Int J Mol Sci. 2023 Sep 22;24(19):14417. doi: 10.3390/ijms241914417. Int J Mol Sci. 2023. PMID: 37833873 Free PMC article.
References
-
- Barry FP, Boynton RE, Haynesworth S, Murphy JM, Zaia J (1999) The monoclonal antibody SH-2, raised against human mesenchymal stem cells, recognizes an epitope on endoglin (CD105). Biochem Biophys Res Commun 265: 134–139 - PubMed
-
- Bos R, van Diest PJ, de Jong JS, van der Groep P, van der Valk P, van der Wall E (2005) Hypoxia-inducible factor-1alpha is associated with angiogenesis, and expression of bFGF, PDGF-BB, and EGFR in invasive breast cancer. Histopathology 46: 31–36 - PubMed
-
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M (2003) Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 92: 692–699 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

