Implementing a system of quality-of-life diagnosis and therapy for breast cancer patients: results of an exploratory trial as a prerequisite for a subsequent RCT
- PMID: 18665187
- PMCID: PMC2527812
- DOI: 10.1038/sj.bjc.6604505
Implementing a system of quality-of-life diagnosis and therapy for breast cancer patients: results of an exploratory trial as a prerequisite for a subsequent RCT
Abstract
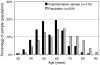
A system for quality-of-life diagnosis and therapy (QoL system) was implemented for breast cancer patients. The system fulfilled the criteria for complex interventions (Medical Research Council). Following theory and modeling, this study contains the exploratory trial as a next step before the randomised clinical trial (RCT) answering three questions: (1) Are there differences between implementation sample and general population? (2) Which amount and type of disagreement exist between patient and coordinating practitioners (CPs) in assessed global QoL? (3) Are there empirical reasons for a cutoff of 50 points discriminating between healthy and diseased QoL? Implementation was successful: 74% of CPs worked along the care pathway. However, CPs showed preferences for selecting patients with lower age and UICC prognostic staging. Patients and CPs disagreed considerably in values of global QoL, despite education in QoL assessment by outreach visits, opinion leaders and CME: Zero values of QoL were only expressed by patients. Finally, the cutoff of 50 points was supported by the relationship between QoL in single items and global QoL: no patients with values above 50 dropped global QoL below 50, but values below 50 and especially at 0 points in single items, induced a dramatic fall of global QoL down to below 50. The exploratory trial was important for defining the complex intervention in the definitive RCT: control for age and prognostic stage grading, support for a QoL unit combining patient's and CP's assessment of QoL and support for the 50-point cutoff criterion between healthy and diseased QoL.
Figures



 case with the worst prognosis, but maximum global QoL.
case with the worst prognosis, but maximum global QoL.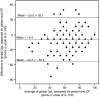


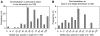
References
-
- Bland J, Altman D (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307–310 - PubMed
-
- Cicchetti D, Sparrow S (1981) Developing criteria for establishing interrater reliability of specific items: applications to assessment of adaptive behavior. Am J Ment Defic 86: 127–137 - PubMed
-
- Engel J, Nagel G, Breuer E, Meisner C, Albert U, Strelocke K, Sauer H, Katenkamp D, Mittermayer Ch, Heidemann E, Schulz K-D, Kunath H, Lorenz W, Hölzel D (2002) Primary breast cancer therapy in six regions of Germany. Eur J Cancer 38: 578–585 - PubMed
-
- Festinger L (1954) A theory of social comparison processes. Hum Relat 7: 117–140
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

