Enzastaurin inhibits tumours sensitive and resistant to anti-EGFR drugs
- PMID: 18665191
- PMCID: PMC2527788
- DOI: 10.1038/sj.bjc.6604493
Enzastaurin inhibits tumours sensitive and resistant to anti-EGFR drugs
Abstract
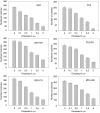
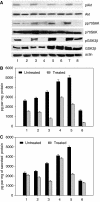
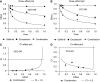
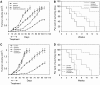
We investigated the antitumour effect and ability to overcome the resistance to anti-EGFR drugs of enzastaurin, an inhibitor of VEGFR-dependent PKCbeta signalling. Enzastaurin was evaluated alone and in combination with the EGFR inhibitor gefitinib, on growth and signalling protein expression in human cancer cells sensitive and resistant to anti-EGFR drugs, both in vitro and in nude mice. We demonstrated the marked inhibitory activity of enzastaurin against GEO colon and PC3 prostate cancer cells and their gefitinib-resistant counterparts GEO-GR and PC3-GR, accompanied by inhibition of pAkt and its effector pp70S6K, pGSK3beta and VEGF expression and secretion. Moreover, enzastaurin showed a cooperative effect with gefitinib in parental and in gefitinib-resistant cells. Remarkably, these results were confirmed in vivo, where enzastaurin showed antitumour activity and cooperativity with gefitinib in mice grafted with GEO and GEO-GR tumours, incrementing their median survival and inhibiting the aforesaid protein expression and secretion in tumour specimens. In conclusion, enzastaurin by interfering with signalling proteins implicated in EGFR drug resistance markedly cooperates with gefitinib in sensitive and gefitinib-resistant tumours, thus overcoming and reverting such resistance and providing a rational basis for its development in patients resistant to anti-EGFR drugs.
Figures
References
-
- Aeder SE, Martin PM, Soh JW, Hussaini IM (2004) PKC-eta mediates glioblastoma cell proliferation through the Akt and mTOR signaling pathways. Oncogene 23: 9062–9069 - PubMed
-
- Beerepoot L, Rademaker-Lakhai J, Witteveen E, Radema S, Viseren-Grul C, Musib L, Van Hal J, Beijnen J, Schellens J, Voest E (2007) Phase I and pharmacokinetic evaluation of enzastaurin combined with gemcitabine and cisplatin in advanced cancer. Clin Cancer Res 13: 4474–4481 - PubMed
-
- Bianco R, Shin I, Ritter CA, Yakes FM, Basso A, Rosen N, Tsurutani J, Dennis PA, Mills GB, Arteaga CL (2003) Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene 22: 2812–2822 - PubMed
-
- Carducci MA, Musib L, Kies MS, Pili R, Truong M, Brahmer JR, Cole P, Sullivan R, Riddle J, Schmidt J, Enas N, Sinha V, Thornton DE, Herbst RS (2006) Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol 24: 4092–4099 - PubMed
-
- Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22: 27–55 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

