Protection induced by Plasmodium falciparum MSP1(42) is strain-specific, antigen and adjuvant dependent, and correlates with antibody responses
- PMID: 18665258
- PMCID: PMC2474699
- DOI: 10.1371/journal.pone.0002830
Protection induced by Plasmodium falciparum MSP1(42) is strain-specific, antigen and adjuvant dependent, and correlates with antibody responses
Abstract
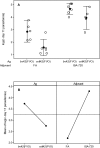
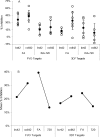
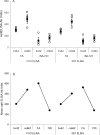
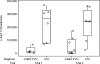
Vaccination with Plasmodium falciparum MSP1(42)/complete Freund's adjuvant (FA) followed by MSP1(42)/incomplete FA is the only known regimen that protects Aotus nancymaae monkeys against infection by erythrocytic stage malaria parasites. The role of adjuvant is not defined; however complete FA cannot be used in humans. In rodent models, immunity is strain-specific. We vaccinated Aotus monkeys with the FVO or 3D7 alleles of MSP1(42) expressed in Escherichia coli or with the FVO allele expressed in baculovirus (bv) combined with complete and incomplete FA, Montanide ISA-720 (ISA-720) or AS02A. Challenge with FVO strain P. falciparum showed that suppression of cumulative day 11 parasitemia was strain-specific and could be induced by E. coli expressed MSP1(42) in combination with FA or ISA-720 but not with AS02A. The coli42-FVO antigen induced a stronger protective effect than the bv42-FVO antigen, and FA induced a stronger protective effect than ISA-720. ELISA antibody (Ab) responses at day of challenge (DOC) were strain-specific and correlated inversely with c-day 11 parasitemia (r = -0.843). ELISA Ab levels at DOC meeting a titer of at least 115,000 ELISA Ab units identified the vaccinees not requiring treatment (noTx) with a true positive rate of 83.3% and false positive rate of 14.3 %. Correlation between functional growth inhibitory Ab levels (GIA) and cumulative day 11 parasitemia was weaker (r = -0.511), and was not as predictive for a response of noTx. The lowest false positive rate for GIA was 30% when requiring a true positive rate of 83.3%. These inhibition results along with those showing that antigen/FA combinations induced a stronger protective immunity than antigen/ISA-720 or antigen/AS02 combinations are consistent with protection as ascribed to MSP1-specific cytophilic antibodies. Development of an effective MSP1(42) vaccine against erythrocytic stage P. falciparum infection will depend not only on antigen quality, but also upon the selection of an optimal adjuvant component.
Conflict of interest statement
Figures


References
-
- Stoute JA, Slaoui M, Heppner DG, Momin P, Kester KE, et al. A preliminary evaluation of a recombinant circumsporozoite protein vaccine against Plasmodium falciparum malaria. RTS,S Malaria Vaccine Evaluation Group. N Engl J Med. 1997;336:86–91. - PubMed
-
- Cohen S, McGregor IA, Carrington S. Gamma globulin and acquired immunity to malaria. Nature. 1964;192:733–737. - PubMed
-
- Diggs CL, Ballou WR, Miller LH. The major merozoite surface protein as malaria vaccine target. Parasitol Today. 1993;9:300–302. - PubMed
-
- Haldar K, Ferguson MA, Cross GA. Acylation of a Plasmodium falciparum merozoite surface antigen via sn- 1,2-diacyl glycerol. J Biol Chem. 1985;260:4969–4974. - PubMed
-
- Holder AA, Freeman RR. Protective antigens of rodent and human bloodstage malaria. Philos Trans R Soc Lond B Biol Sci. 1984;307:171–177. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

