Prevalence and infection load dynamics of Rickettsia felis in actively feeding cat fleas
- PMID: 18665265
- PMCID: PMC2474969
- DOI: 10.1371/journal.pone.0002805
Prevalence and infection load dynamics of Rickettsia felis in actively feeding cat fleas
Abstract
Background: Rickettsia felis is a flea-associated rickettsial pathogen recurrently identified in both colonized and wild-caught cat fleas, Ctenocephalides felis. We hypothesized that within colonized fleas, the intimate relationship between R. felis and C. felis allows for the coordination of rickettsial replication and metabolically active periods during flea bloodmeal acquisition and oogenesis.
Methodology/principal findings: A quantitative real-time PCR assay was developed to quantify R. felis in actively feeding R. felis-infected fleas. In three separate trials, fleas were allowed to feed on cats, and a mean of 3.9x10(6) R. felis 17-kDa gene copies was detected for each flea. A distinct R. felis infection pattern was not observed in fleas during nine consecutive days of bloodfeeding. However, an inverse correlation between the prevalence of R. felis-infection, which ranged from 96% in Trial 1 to 35% in Trial 3, and the R. felis-infection load in individual fleas was identified. Expression of R. felis-infection load as a ratio of R. felis/C. felis genes confirmed that fleas in Trial 3 had significantly greater rickettsial loads than those in Trial 1.
Conclusion/significance: Examining rickettsial infection dynamics in the flea vector will further elucidate the intimate relationship between R. felis and C. felis, and facilitate a more accurate understanding of the ecology and epidemiology of R. felis transmission in nature.
Conflict of interest statement
Figures
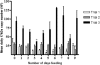



Similar articles
-
Effect of Rickettsia felis Strain Variation on Infection, Transmission, and Fitness in the Cat Flea (Siphonaptera: Pulicidae).J Med Entomol. 2017 Jul 1;54(4):1037-1043. doi: 10.1093/jme/tjx046. J Med Entomol. 2017. PMID: 28399259 Free PMC article.
-
Horizontal transmission of Rickettsia felis between cat fleas, Ctenocephalides felis.Mol Ecol. 2011 Nov;20(21):4577-86. doi: 10.1111/j.1365-294X.2011.05289.x. Epub 2011 Oct 4. Mol Ecol. 2011. PMID: 21967477 Free PMC article.
-
Dissemination of bloodmeal acquired Rickettsia felis in cat fleas, Ctenocephalides felis.Parasit Vectors. 2013 May 24;6:149. doi: 10.1186/1756-3305-6-149. Parasit Vectors. 2013. PMID: 23705666 Free PMC article.
-
Ecology of Rickettsia felis: a review.J Med Entomol. 2009 Jul;46(4):723-36. doi: 10.1603/033.046.0402. J Med Entomol. 2009. PMID: 19645274 Review.
-
Prevalence of Rickettsia felis DNA in the blood of cats and their fleas in the United States.J Feline Med Surg. 2007 Jun;9(3):258-62. doi: 10.1016/j.jfms.2006.12.005. Epub 2007 Feb 2. J Feline Med Surg. 2007. PMID: 17276123 Free PMC article. Review.
Cited by
-
Heme binding proteins of Bartonella henselae are required when undergoing oxidative stress during cell and flea invasion.PLoS One. 2012;7(10):e48408. doi: 10.1371/journal.pone.0048408. Epub 2012 Oct 29. PLoS One. 2012. PMID: 23144761 Free PMC article.
-
Acquisition of Rickettsia felis by cat fleas during feeding.Vector Borne Zoonotic Dis. 2011 Jul;11(7):963-8. doi: 10.1089/vbz.2010.0137. Epub 2011 Jan 9. Vector Borne Zoonotic Dis. 2011. PMID: 21214386 Free PMC article.
-
A reverse vaccinology approach to the identification and characterization of Ctenocephalides felis candidate protective antigens for the control of cat flea infestations.Parasit Vectors. 2018 Jan 18;11(1):43. doi: 10.1186/s13071-018-2618-x. Parasit Vectors. 2018. PMID: 29347954 Free PMC article.
-
Prevalence and burden of two rickettsial phylotypes (G021 and G022) in Ixodes pacificus from California by real-time quantitative PCR.Ticks Tick Borne Dis. 2013 Jun;4(4):280-7. doi: 10.1016/j.ttbdis.2012.12.005. Epub 2013 Mar 22. Ticks Tick Borne Dis. 2013. PMID: 23522936 Free PMC article.
-
Rickettsia felis in Aedes albopictus mosquitoes, Libreville, Gabon.Emerg Infect Dis. 2012 Oct;18(10):1687-9. doi: 10.3201/eid1810.120178. Emerg Infect Dis. 2012. PMID: 23017437 Free PMC article. No abstract available.
References
-
- Adams JR, Schmidtmann ET, Azad AF. Infection of colonized cat fleas, Ctenocephalides felis (Bouche), with a rickettsia-like microorganism. Am J Trop Med Hyg. 1990;43:400–409. - PubMed
-
- Wedincamp J, Jr, Foil LD. Vertical transmission of Rickettsia felis in the cat flea (Ctenocephalides felis Bouche). J Vector Ecol. 2002;27:96–101. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

