Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels
- PMID: 18665391
- PMCID: PMC2802130
- DOI: 10.1007/s00424-008-0550-1
Complex functions of phosphatidylinositol 4,5-bisphosphate in regulation of TRPC5 cation channels
Abstract
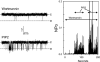
The canonical transient receptor potential (TRPC) proteins have been recognized as key players in calcium entry pathways activated through phospholipase-C-coupled receptors. While it is clearly demonstrated that members of the TRPC3/6/7 subfamily are activated by diacylglycerol, the mechanism by which phospholipase C activates members of the TRPC1/4/5 subfamily remains a mystery. In this paper, we provide evidence for both negative and positive modulatory roles for membrane polyphosphoinositides in the regulation of TRPC5 channels. Depletion of polyphosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate (PIP2) through inhibition of phosphatidylinositol 4-kinase activates calcium entry and membrane currents in TRPC5-expressing but not in TRPC3- or TRPC7-expressing cells. Inclusion of polyphosphatidylinositol 4-phosphate or PIP2, but not phosphatidylinositol 3,4,5-trisphosphate, in the patch pipette inhibited TRPC5 currents. Paradoxically, depletion of PIP2 with a directed 5-phosphatase strategy inhibited TRPC5. Furthermore, when the activity of single TRPC5 channels was examined in excised patches, the channels were robustly activated by PIP2. These findings indicate complex functions for regulation of TRPC5 by PIP2, and we propose that membrane polyphosphoinositides may have at least two distinct functions in regulating TRPC5 channel activity.
Figures
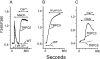




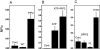



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

