Mitochondrial biogenesis in the axons of vertebrate peripheral neurons
- PMID: 18666204
- PMCID: PMC2538952
- DOI: 10.1002/dneu.20668
Mitochondrial biogenesis in the axons of vertebrate peripheral neurons
Abstract
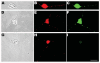
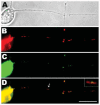
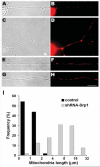
Mitochondria are widely distributed via regulated transport in neurons, but their sites of biogenesis remain uncertain. Most mitochondrial proteins are encoded in the nuclear genome, and evidence has suggested that mitochondrial DNA (mtDNA) replication occurs mainly or entirely in the cell body. However, it has also become clear that nuclear-encoded mitochondrial proteins can be translated in the axon and that components of the mitochondrial replication machinery reside there as well. We assessed directly whether mtDNA replication can occur in the axons of chick peripheral neurons labeled with 5-bromo-2'-deoxyuridine (BrdU). In axons that were physically separated from the cell body or had disrupted organelle transport between the cell bodies and axons, a significant fraction of mtDNA synthesis continued. We also detected the mitochondrial fission protein Drp1 in neurons by immunofluorescence or expression of GFP-Drp1. Its presence and distribution on the majority of axonal mitochondria indicated that a substantial number had undergone recent division in the axon. Because the morphology of mitochondria is maintained by the balance of fission and fusion events, we either inhibited Drp1 expression by RNAi or overexpressed the fusion protein Mfn1. Both methods resulted in significantly longer mitochondria in axons, including many at a great distance from the cell body. These data indicate that mitochondria can replicate their DNA, divide, and fuse locally within the axon; thus, the biogenesis of mitochondria is not limited to the cell body.
Copyright (c) 2008 Wiley Periodicals, Inc.
Figures




References
-
- Alvarez J, Giuditta A, Koenig E. Protein synthesis in axons and terminals: Significance for maintenance, plasticity and regulation of phenotype. With a critique of slow transport theory. Prog Neurobiol. 2000;62:1–62. - PubMed
-
- Berthold CH, Fabricius C, Rydmark M, Andersen B. Axoplasmic organelles at nodes of Ranvier. I. Occurrence and distribution in large myelinated spinal root axons of the adult cat. J Neurocytol. 1993;22:925–940. - PubMed
-
- Bristow EA, Griffiths PG, Andrews RM, Johnson MA, Turnbull DM. The distribution of mitochondrial activity in relation to optic nerve structure. Arch Ophthalmol. 2002;120:791–796. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

