Efficacy of 6-mercaptopurine treatment after azathioprine hypersensitivity in inflammatory bowel disease
- PMID: 18666323
- PMCID: PMC2731186
- DOI: 10.3748/wjg.14.4342
Efficacy of 6-mercaptopurine treatment after azathioprine hypersensitivity in inflammatory bowel disease
Abstract
Aim: To investigate the efficacy of 6-mercaptopurine (6-MP) in cases of azathioprine (AZA) hypersensitivity in patients with inflammatory bowel disease.
Methods: Twenty nine previously confirmed Crohn's disease (CD) (n = 14) and ulcerative colitis (UC) (n = 15) patients with a known previous (AZA) hypersensitivity reaction were studied prospectively. The 6-MP doses were gradually increased from 0.5 up to 1.0-1.5 mg/kg per day. Clinical activity indicies (CDAI/CAI), laboratory variables and daily doses of oral 5-ASA, corticosteroids, and 6-MP were assessed before and in the first, sixth and twelfth months of treatment.
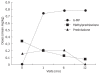
Results: In 9 patients, 6-MP was withdrawn in the first 2 wk due to an early hypersensitivity reaction. Medication was ineffective within 6 mo in 6 CD patients, and myelotoxic reaction was observed in two. Data were evaluated at the end of the sixth month in 12 (8 UC, 4 CD) patients, and after the first year in 9 (6 UC, 3 CD) patients. CDAI decreased transiently at the end of the sixth month, but no significant changes were observed in the CDAI or the CAI values at the end of the year. Leukocyte counts (P = 0.01), CRP (P = 0.02), and serum iron (P = 0.05) values indicated decreased inflammatory reactions, especially in the UC patients at the end of the year, making the possibility to taper oral steroid doses.
Conclusion: About one-third of the previously AZA-intolerant patients showed adverse effects on taking 6MP. In our series, 20 patients tolerated 6MP, but it was ineffective in 8 CD cases, and valuable mainly in ulcerative colitis patients.
Figures
Similar articles
-
Efficacy of methotrexate in Crohn's disease and ulcerative colitis patients unresponsive or intolerant to azathioprine /mercaptopurine.Aliment Pharmacol Ther. 2009 Sep 15;30(6):614-20. doi: 10.1111/j.1365-2036.2009.04073.x. Epub 2009 Jun 23. Aliment Pharmacol Ther. 2009. PMID: 19552632
-
Birth outcome in women with ulcerative colitis and Crohn's disease, and pharmacoepidemiological aspects of anti-inflammatory drug therapy.Dan Med Bull. 2011 Dec;58(12):B4360. Dan Med Bull. 2011. PMID: 22142578
-
Tolerability and safety of mercaptopurine in azathioprine-intolerant patients with inflammatory bowel disease.Aliment Pharmacol Ther. 2008 Feb 1;27(3):220-7. doi: 10.1111/j.1365-2036.2007.03570.x. Epub 2007 Nov 6. Aliment Pharmacol Ther. 2008. PMID: 17988235
-
Conventional therapy for moderate to severe inflammatory bowel disease: A systematic literature review.World J Gastroenterol. 2019 Mar 7;25(9):1142-1157. doi: 10.3748/wjg.v25.i9.1142. World J Gastroenterol. 2019. PMID: 30863001 Free PMC article.
-
6-Mercaptopurine for Azathioprine Intolerant Inflammatory Bowel Disease: Literature Search and Reappraisal of Own Data.Inflamm Allergy Drug Targets. 2015;14(2):133-7. doi: 10.2174/1871528114666160105112915. Inflamm Allergy Drug Targets. 2015. PMID: 26728773 Review.
Cited by
-
Updates on conventional therapies for inflammatory bowel diseases: 5-aminosalicylates, corticosteroids, immunomodulators, and anti-TNF-α.Korean J Intern Med. 2022 Sep;37(5):895-905. doi: 10.3904/kjim.2022.132. Epub 2022 Jul 27. Korean J Intern Med. 2022. PMID: 35882566 Free PMC article. Review.
-
Optimizing the use of thiopurines in inflammatory bowel disease.Ther Adv Chronic Dis. 2015 May;6(3):138-46. doi: 10.1177/2040622315579063. Ther Adv Chronic Dis. 2015. PMID: 25954498 Free PMC article. Review.
-
Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease.World J Gastroenterol. 2011 Oct 7;17(37):4166-73. doi: 10.3748/wjg.v17.i37.4166. World J Gastroenterol. 2011. PMID: 22072847 Free PMC article. Review.
-
Use of Azathioprine in Ulcerative Colitis: A Comprehensive Review.Cureus. 2022 May 10;14(5):e24874. doi: 10.7759/cureus.24874. eCollection 2022 May. Cureus. 2022. PMID: 35698683 Free PMC article. Review.
References
-
- Nielsen OH, Munck LK. Drug insight: aminosalicylates for the treatment of IBD. Nat Clin Pract Gastroenterol Hepatol. 2007;4:160–170. - PubMed
-
- Goldstein F. Immunosuppressant therapy of inflammatory bowel disease. Pharmacologic and clinical aspects. J Clin Gastroenterol. 1987;9:654–658. - PubMed
-
- Katz JA. Advances in the medical therapy of inflammatory bowel disease. Curr Opin Gastroenterol. 2002;18:435–440. - PubMed
-
- Gilissen LP, Derijks LJ, Bos LP, Bus PJ, Hooymans PM, Engels LG. Therapeutic drug monitoring in patients with inflammatory bowel disease and established azathioprine therapy. Clin Drug Investig. 2004;24:479–486. - PubMed
-
- Robinson M. Optimizing therapy for inflammatory bowel disease. Am J Gastroenterol. 1997;92:12S–17S. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous