Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naïve populations and associate with reduced treatment efficacy
- PMID: 18666824
- PMCID: PMC2488194
- DOI: 10.1371/journal.pmed.0050158
Minority HIV-1 drug resistance mutations are present in antiretroviral treatment-naïve populations and associate with reduced treatment efficacy
Abstract
Background: Transmitted HIV-1 drug resistance can compromise initial antiretroviral therapy (ART); therefore, its detection is important for patient management. The absence of drug-associated selection pressure in treatment-naïve persons can cause drug-resistant viruses to decline to levels undetectable by conventional bulk sequencing (minority drug-resistant variants). We used sensitive and simple tests to investigate evidence of transmitted drug resistance in antiretroviral drug-naïve persons and assess the clinical implications of minority drug-resistant variants.
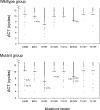
Methods and findings: We performed a cross-sectional analysis of transmitted HIV-1 drug resistance and a case-control study of the impact of minority drug resistance on treatment response. For the cross-sectional analysis, we examined viral RNA from newly diagnosed ART-naïve persons in the US and Canada who had no detectable (wild type, n = 205) or one or more resistance-related mutations (n = 303) by conventional sequencing. Eight validated real-time PCR-based assays were used to test for minority drug resistance mutations (protease L90M and reverse transcriptase M41L, K70R, K103N, Y181C, M184V, and T215F/Y) above naturally occurring frequencies. The sensitive real-time PCR testing identified one to three minority drug resistance mutation(s) in 34/205 (17%) newly diagnosed persons who had wild-type virus by conventional genotyping; four (2%) individuals had mutations associated with resistance to two drug classes. Among 30/303 (10%) samples with bulk genotype resistance mutations we found at least one minority variant with a different drug resistance mutation. For the case-control study, we assessed the impact of three treatment-relevant drug resistance mutations at baseline from a separate group of 316 previously ART-naïve persons with no evidence of drug resistance on bulk genotype testing who were placed on efavirenz-based regimens. We found that 7/95 (7%) persons who experienced virologic failure had minority drug resistance mutations at baseline; however, minority resistance was found in only 2/221 (0.9%) treatment successes (Fisher exact test, p = 0.0038).
Conclusions: These data suggest that a considerable proportion of transmitted HIV-1 drug resistance is undetected by conventional genotyping and that minority mutations can have clinical consequences. With no treatment history to help guide therapies for drug-naïve persons, the findings suggest an important role for sensitive baseline drug resistance testing.
Conflict of interest statement
Figures


Comment in
-
Transmitted minority drug-resistant HIV variants: a new epidemic?PLoS Med. 2008 Jul 29;5(7):e164. doi: 10.1371/journal.pmed.0050164. PLoS Med. 2008. PMID: 18666826 Free PMC article.
References
-
- Weinstock HS, Zaidi I, Heneine W, Bennett D, Garcia-Lerma JG, et al. The epidemiology of antiretroviral drug resistance among drug-naïve HIV-1 infected persons in 10 U.S. cities. J Infect Dis. 2004;189:174–180. - PubMed
-
- Grant RM, Hecht FM, Warmerdam M, Liu L, Liegler T, et al. Time trends in primary HIV-1 drug resistance among recently infected persons. JAMA. 2002;288:181–188. - PubMed
-
- Jayaraman GC, Archibald CP, Kim J, Rekart ML, Singh AE, et al. A population-based approach to determine the prevalence of transmitted drug-resistant HIV among recent versus established HIV infections: results from the Canadian HIV strain and drug resistance surveillance program. J Acquir Immune Defic Syndr. 2006;42:86–90. - PubMed
-
- Little SJ, Holte S, Routy JP, Daar ES, Markowitz M, et al. Antiretroviral-drug resistance among patients recently infected with HIV. N Engl J Med. 2002;347:385–394. - PubMed
-
- Truong HH, Grant RM, McFarland W, Kellogg T, Kent C, et al. Routine surveillance for the detection of acute and recent HIV infections and transmission of antiretroviral resistance. AIDS. 2006;20:2193–2197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

