Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium
- PMID: 18667074
- PMCID: PMC2518925
- DOI: 10.1186/1471-213X-8-75
Msx1 and Msx2 are required for endothelial-mesenchymal transformation of the atrioventricular cushions and patterning of the atrioventricular myocardium
Abstract
Background: Msx1 and Msx2, which belong to the highly conserved Nk family of homeobox genes, display overlapping expression patterns and redundant functions in multiple tissues and organs during vertebrate development. Msx1 and Msx2 have well-documented roles in mediating epithelial-mesenchymal interactions during organogenesis. Given that both Msx1 and Msx2 are crucial downstream effectors of Bmp signaling, we investigated whether Msx1 and Msx2 are required for the Bmp-induced endothelial-mesenchymal transformation (EMT) during atrioventricular (AV) valve formation.
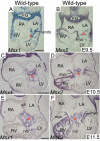
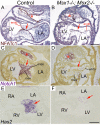
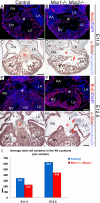
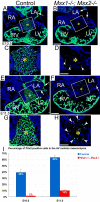
Results: While both Msx1-/- and Msx2-/- single homozygous mutant mice exhibited normal valve formation, we observed hypoplastic AV cushions and malformed AV valves in Msx1-/-; Msx2-/- mutants, indicating redundant functions of Msx1 and Msx2 during AV valve morphogenesis. In Msx1/2 null mutant AV cushions, we found decreased Bmp2/4 and Notch1 signaling as well as reduced expression of Has2, NFATc1 and Notch1, demonstrating impaired endocardial activation and EMT. Moreover, perturbed expression of chamber-specific genes Anf, Tbx2, Hand1 and Hand2 reveals mispatterning of the Msx1/2 double mutant myocardium and suggests functions of Msx1 and Msx2 in regulating myocardial signals required for remodelling AV valves and maintaining an undifferentiated state of the AV myocardium.
Conclusion: Our findings demonstrate redundant roles of Msx1 and Msx2 in regulating signals required for development of the AV myocardium and formation of the AV valves.
Figures



Similar articles
-
Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning.Development. 2005 Dec;132(24):5601-11. doi: 10.1242/dev.02156. Development. 2005. PMID: 16314491
-
Msx1 and Msx2 regulate survival of secondary heart field precursors and post-migratory proliferation of cardiac neural crest in the outflow tract.Dev Biol. 2007 Aug 15;308(2):421-37. doi: 10.1016/j.ydbio.2007.05.037. Epub 2007 Jun 4. Dev Biol. 2007. PMID: 17601530
-
Expression of muscle segment homeobox genes in the developing myocardium.Anat Rec (Hoboken). 2010 Jun;293(6):998-1001. doi: 10.1002/ar.21112. Anat Rec (Hoboken). 2010. PMID: 20225205
-
Nfatc1 directs the endocardial progenitor cells to make heart valve primordium.Trends Cardiovasc Med. 2013 Nov;23(8):294-300. doi: 10.1016/j.tcm.2013.04.003. Epub 2013 May 10. Trends Cardiovasc Med. 2013. PMID: 23669445 Free PMC article. Review.
-
Transcriptional regulation of heart valve progenitor cells.Pediatr Cardiol. 2010 Apr;31(3):414-21. doi: 10.1007/s00246-009-9616-x. Epub 2009 Dec 29. Pediatr Cardiol. 2010. PMID: 20039031 Free PMC article. Review.
Cited by
-
CCBE1 Is Essential for Epicardial Function during Myocardium Development.Int J Mol Sci. 2022 Oct 20;23(20):12642. doi: 10.3390/ijms232012642. Int J Mol Sci. 2022. PMID: 36293499 Free PMC article.
-
Regulation of MDM2 E3 ligase-dependent vascular calcification by MSX1/2.Exp Mol Med. 2021 Nov;53(11):1781-1791. doi: 10.1038/s12276-021-00708-6. Epub 2021 Nov 29. Exp Mol Med. 2021. PMID: 34845330 Free PMC article.
-
Epidemiological and clinical evaluation of patients with a cleft in lower saxony Germany: a mono-center analysis.Clin Oral Investig. 2023 Sep;27(9):5661-5670. doi: 10.1007/s00784-023-05187-9. Epub 2023 Aug 5. Clin Oral Investig. 2023. PMID: 37542681 Free PMC article.
-
Myocardial β-Catenin-BMP2 signaling promotes mesenchymal cell proliferation during endocardial cushion formation.J Mol Cell Cardiol. 2018 Oct;123:150-158. doi: 10.1016/j.yjmcc.2018.09.001. Epub 2018 Sep 8. J Mol Cell Cardiol. 2018. PMID: 30201295 Free PMC article.
-
MSX1 inhibits cell migration and invasion through regulating the Wnt/β-catenin pathway in glioblastoma.Tumour Biol. 2016 Jan;37(1):1097-104. doi: 10.1007/s13277-015-3892-2. Epub 2015 Aug 15. Tumour Biol. 2016. PMID: 26271668
References
-
- Schroeder JA, Jackson LF, Lee DC, Camenisch TD. Form and function of developing heart valves: coordination by extracellular matrix and growth factor signaling. J Mol Med. 2003;81:392–403. - PubMed
-
- Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. - PubMed
-
- Zaffran S, Frasch M. Early signals in cardiac development. Circ Res. 2002;91:457–469. - PubMed
-
- Eisenberg LM, Markwald RR. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

