Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks
- PMID: 18667150
- PMCID: PMC2578820
- DOI: 10.1016/j.neuron.2008.06.008
Abeta plaques lead to aberrant regulation of calcium homeostasis in vivo resulting in structural and functional disruption of neuronal networks
Abstract
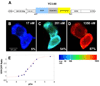
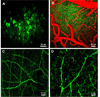
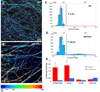
Alzheimer's disease is characterized by the deposition of senile plaques and progressive dementia. The molecular mechanisms that couple plaque deposition to neural system failure, however, are unknown. Using transgenic mouse models of AD together with multiphoton imaging, we measured neuronal calcium in individual neurites and spines in vivo using the genetically encoded calcium indicator Yellow Cameleon 3.6. Quantitative imaging revealed elevated [Ca(2+)]i (calcium overload) in approximately 20% of neurites in APP mice with cortical plaques, compared to less than 5% in wild-type mice, PS1 mutant mice, or young APP mice (animals without cortical plaques). Calcium overload depended on the existence and proximity to plaques. The downstream consequences included the loss of spinodendritic calcium compartmentalization (critical for synaptic integration) and a distortion of neuritic morphologies mediated, in part, by the phosphatase calcineurin. Together, these data demonstrate that senile plaques impair neuritic calcium homeostasis in vivo and result in the structural and functional disruption of neuronal networks.
Figures





References
-
- Akbari Y, Hitt BD, Murphy MP, Dagher NN, Tseng BP, Green KN, Golde TE, LaFerla FM. Presenilin regulates capacitative calcium entry dependently and independently of gamma-secretase activity. Biochem Biophys Res Commun. 2004;322:1145–1152. - PubMed
-
- Bacskai BJ, Klunk WE, Mathis CA, Hyman BT. Imaging amyloidbeta deposits in vivo. J Cereb Blood Flow Metab. 2002;22:1035–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

