Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab
- PMID: 18667396
- PMCID: PMC2733116
- DOI: 10.1093/annonc/mdn427
Hormone receptor status and pathologic response of HER2-positive breast cancer treated with neoadjuvant chemotherapy and trastuzumab
Abstract
Background: The aim of this study was to compare the extent of pathologic response in patients with HER2-positive (HER2+) breast cancer treated with standard neoadjuvant chemotherapy, with or without trastuzumab (H), according to hormone receptor (HR) status.
Patients and methods: We included 199 patients with HER2+ breast cancer from three successive cohorts of neo-adjuvant chemotherapy on the basis of paclitaxel (Taxol) (P) administered weekly (w) or three weekly (3-w), followed by 5-fluorouracil (F), doxorubicin (A) or epirubicin (E), and cyclophosphamide (C). Residual cancer burden (RCB) was determined from pathologic review of the primary tumor and lymph nodes and was classified as pathologic complete response (pCR) or minimal (RCB-I), moderate (RCB-II), or extensive (RCB-III) residual disease.
Results: In HR-positive (HR+) cancers, a higher rate of pathologic response (pCR/RCB-I) was observed with concurrent H + 3-wP/FEC (73%) than with 3-wP/FEC (34%, P = 0.002) or wP/FAC (47%; P = 0.02) chemotherapy alone. In HR-negative (HR-) cancers, there were no significant differences in the rate of pathologic response (pCR/RCB-I) from 3-wP/FAC (50%), wP/FAC (68%), or concurrent H + 3-wP/FEC (72%).
Conclusions: Patients with HR+/HER2+ breast cancer obtained significant benefit from addition of trastuzumab to P/FEC chemotherapy; pathologic response rate was similar to that seen in HR-/HER2+ breast cancers.
Figures


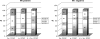
References
-
- Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Marty M, Cognetti F, Maraninchi D, et al. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. - PubMed
-
- Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. - PubMed
-
- Buzdar AU, Valero V, Ibrahim NK, et al. Neoadjuvant therapy with paclitaxel followed by 5-fluorouracil, epirubicin, and cyclophosphamide chemotherapy and concurrent trastuzumab in human epidermal growth factor receptor 2-positive operable breast cancer: an update of the initial randomized study population and data of additional patients treated with the same regimen. Clin Cancer Res. 2007;13:228–233. - PubMed
-
- Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

