Potent antibody-mediated neutralization and evolution of antigenic escape variants of simian immunodeficiency virus strain SIVmac239 in vivo
- PMID: 18667507
- PMCID: PMC2546989
- DOI: 10.1128/JVI.00871-08
Potent antibody-mediated neutralization and evolution of antigenic escape variants of simian immunodeficiency virus strain SIVmac239 in vivo
Abstract
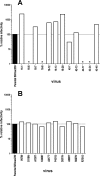
Here, we describe the evolution of antigenic escape variants in a rhesus macaque that developed unusually high neutralizing antibody titers to SIVmac239. By 42 weeks postinfection, 50% neutralization of SIVmac239 was achieved with plasma dilutions of 1:1,000. Testing of purified immunoglobulin confirmed that the neutralizing activity was antibody mediated. Despite the potency of the neutralizing antibody response, the animal displayed a typical viral load profile and progressed to terminal AIDS with a normal time course. Viral envelope sequences from week 16 and week 42 plasma contained an excess of nonsynonymous substitutions, predominantly in V1 and V4, including individual sites with ratios of nonsynonymous to synonymous substitution rates (dN/dS) highly suggestive of strong positive selection. Recombinant viruses encoding envelope sequences isolated from these time points remained resistant to neutralization by all longitudinal plasma samples, revealing the failure of the animal to mount secondary responses to the escaped variants. Substitutions at two sites with significant dN/dS values, one in V1 and one in V4, were independently sufficient to confer nearly complete resistance to neutralization. Substitutions at three additional sites, one in V4 and two in gp41, conferred moderate to high levels of resistance when tested individually. All the amino acid changes leading to escape resulted from single nucleotide substitutions. The observation that antigenic escape resulted from individual, single amino acid replacements at sites well separated in current structural models of Env indicates that the virus can utilize multiple independent pathways to rapidly achieve similar levels of resistance.
Figures

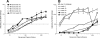






References
-
- Anisimova, M., J. P. Bielawski, and Z. Yang. 2002. Accuracy and power of Bayes prediction of amino acid sites under positive selection. Mol. Biol. Evol. 19950-958. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

