Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating
- PMID: 18667513
- PMCID: PMC2546945
- DOI: 10.1128/JVI.01014-08
Caveolin-1-dependent infectious entry of human papillomavirus type 31 in human keratinocytes proceeds to the endosomal pathway for pH-dependent uncoating
Abstract
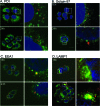
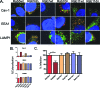
High-risk human papillomaviruses (HPVs) are small nonenveloped DNA viruses with a strict tropism for squamous epithelium. The viruses are causative agents of cervical cancer and some head and neck cancers, but their differentiation-dependent life cycles have made them difficult to study in simple cell culture. Thus, many aspects of early HPV infection remain mysterious. We recently showed the high-risk HPV type 31 (HPV31) enters its natural host cell type via caveola-dependent endocytosis, a distinct mechanism from that of the closely related HPV16 (Smith et al., J. Virol. 81:9922-9931, 2007). Here, we determined the downstream trafficking events after caveolar entry of HPV31 into human keratinocytes. After initial plasma membrane binding, HPV31 associates with caveolin-1 and transiently localizes to the caveosome before trafficking to the early endosome and proceeding through the endosomal pathway. Caveosome-to-endosome transport was found to be Rab5 GTPase dependent. Although HPV31 capsids were observed in the lysosome, Rab7 GTPase was dispensable for HPV31 infection, suggesting that viral genomes escape from the endosomal pathway prior to Rab7-mediated capsid transport. Consistent with this, the acidic pH encountered by HPV31 within the early endosomal pathway induces a conformational change in the capsid resulting in increased DNase susceptibility of the viral genome, which likely aids in uncoating and/or endosomal escape. The entry and trafficking route of HPV31 into human keratinocytes represents a unique viral pathway by which the virions use caveolar entry to eventually access a low-pH site that appears to facilitate endosomal escape of genomes.
Figures



References
-
- Barr, E., C. K. Gause, O. M. Bautista, R. A. Railkar, L. C. Lupinacci, R. P. Insinga, H. L. Sings, and R. M. Haupt. 2008. Impact of a prophylactic quadrivalent human papillomavirus (types 6, 11, 16, 18) L1 virus-like particle vaccine in a sexually active population of North American women. Am. J. Obstet. Gynecol. 198261.e1-261.e11. - PubMed
-
- Bosch, F. X., M. M. Manos, N. Muñoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, and I. S. Group. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87796-802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

