Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells
- PMID: 18667516
- PMCID: PMC2566289
- DOI: 10.1128/JVI.01083-08
Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells
Abstract
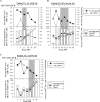
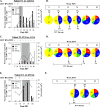
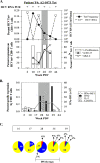
The majority of acute hepatitis C virus (HCV) infections progress to chronicity and progressive liver damage. Alpha interferon (IFN-alpha) antiviral therapy achieves the highest rate of success when IFN-alpha is administered early during the acute phase, but the underlying mechanisms are unknown. We used a panel of major histocompatibility complex class I tetramers to monitor the phenotypic and functional signatures of HCV-specific T cells during acute HCV infection with different infection outcomes and during early IFN therapy. We demonstrate that spontaneous resolution correlates with the early development of polyfunctional (IFN-gamma- and IL-2-producing and CD107a(+)) virus-specific CD8(+) T cells. These polyfunctional T cells are distinguished by the expression of CD127 and Bcl-2 and represent a transitional memory T-cell subset that exhibits the phenotypic and functional signatures of both central and effector memory T cells. In contrast, HCV-specific CD8(+) T cells in acute infections evolving to chronicity expressed low levels of CD127 and Bcl-2, exhibited diminished proliferation and cytokine production, and eventually disappeared from the periphery. Early therapeutic intervention with pegylated IFN-alpha rescued polyfunctional memory T cells expressing high levels of CD127 and Bcl-2. These cells were detectable for up to 1 year following discontinuation of therapy. Our results suggest that the polyfunctionality of HCV-specific T cells can be predictive of the outcome of acute HCV infection and that early therapeutic intervention can reconstitute the pool of long-lived polyfunctional memory T cells.
Figures




References
-
- Almeida, J. R., D. A. Price, L. Papagno, Z. A. Arkoub, D. Sauce, E. Bornstein, T. E. Asher, A. Samri, A. Schnuriger, I. Theodorou, D. Costagliola, C. Rouzioux, H. Agut, A. G. Marcelin, D. Douek, B. Autran, and V. Appay. 2007. Superior control of HIV-1 replication by CD8+ T cells is reflected by their avidity, polyfunctionality, and clonal turnover. J. Exp. Med. 2042473-2485. - PMC - PubMed
-
- Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8379-385. - PubMed
-
- Bengsch, B., H. C. Spangenberg, N. Kersting, C. Neumann-Haefelin, E. Panther, F. von Weizsacker, H. E. Blum, H. Pircher, and R. Thimme. 2007. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J. Virol. 81945-953. - PMC - PubMed
-
- Betts, M. R., J. M. Brenchley, D. A. Price, S. C. De Rosa, D. C. Douek, M. Roederer, and R. A. Koup. 2003. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J. Immunol. Methods. 28165-78. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

