Prominent actin fiber arrays in Drosophila tendon cells represent architectural elements different from stress fibers
- PMID: 18667532
- PMCID: PMC2555930
- DOI: 10.1091/mbc.e08-02-0182
Prominent actin fiber arrays in Drosophila tendon cells represent architectural elements different from stress fibers
Abstract
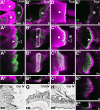
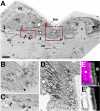
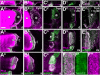
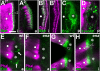
Tendon cells are specialized cells of the insect epidermis that connect basally attached muscle tips to the cuticle on their apical surface via prominent arrays of microtubules. Tendon cells of Drosophila have become a useful genetic model system to address questions with relevance to cell and developmental biology. Here, we use light, confocal, and electron microscopy to present a refined model of the subcellular organization of tendon cells. We show that prominent arrays of F-actin exist in tendon cells that fully overlap with the microtubule arrays, and that type II myosin accumulates in the same area. The F-actin arrays in tendon cells seem to represent a new kind of actin structure, clearly distinct from stress fibers. They are highly resistant to F-actin-destabilizing drugs, to the application of myosin blockers, and to loss of integrin, Rho1, or mechanical force. They seem to represent an important architectural element of tendon cells, because they maintain a connection between apical and basal surfaces even when microtubule arrays of tendon cells are dysfunctional. Features reported here and elsewhere for tendon cells are reminiscent of the structural and molecular features of support cells in the inner ear of vertebrates, and they might have potential translational value.
Figures



References
-
- Adams M. D., et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–2195. - PubMed
-
- Arima T., Uemura T., Yamamoto T. Cytoskeletal organization in the supporting cell of the guinea pig organ of Corti. Hear. Res. 1986;24:169–175. - PubMed
-
- Bartnik E., Weber K. Widespread occurrence of intermediate filaments in invertebrates; common principles and aspects of diversion. Eur. J. Cell Biol. 1989;50:17–33.
-
- Baumgärtner S., Littleton J. T., Broadie K., Bhat M. A., Harbecke R., Lengyel J. A., Chiquet-Ehrismann R., Prokop A., Bellen H. J. A Drosophila neurexin is required for the formation and function of septate junctions. Cell. 1996;87:1059–1068. - PubMed
-
- Bershadsky A. D., Ballestrem C., Carramusa L., Zilberman Y., Gilquin B., Khochbin S., Alexandrova A. Y., Verkhovsky A. B., Shemesh T., Kozlov M. M. Assembly and mechanosensory function of focal adhesions: experiments and models. Eur J. Cell Biol. 2006;85:165–173. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

