NMDA receptor antagonists reveal age-dependent differences in the properties of visual cortical plasticity
- PMID: 18667547
- PMCID: PMC2576203
- DOI: 10.1152/jn.90290.2008
NMDA receptor antagonists reveal age-dependent differences in the properties of visual cortical plasticity
Abstract
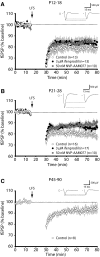
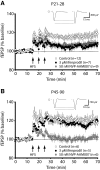
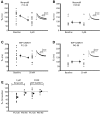
The suggestion that NMDA receptor (NMDAR)-dependent plasticity is subunit specific, with NR2B-types required for long-term depression (LTD) and NR2A-types critical for the induction of long-term potentiation (LTP), has generated much attention and considerable debate. By investigating the suggested subunit-specific roles of NMDARs in the mouse primary visual cortex over development, we report several important findings that clarify the roles of NMDAR subtypes in synaptic plasticity. We observed that LTD was not attenuated by application of ifenprodil, an NR2B-type antagonist, or NVP-AAM007, a less selective NR2A-type antagonist. However, we were surprised that NVP-AAM007 completely blocked adult LTP (postnatal day (P) 45-90), while only modestly affecting juvenile LTP (P21-28). To assess whether this developmental transition reflected an increasing role for NR2A-type receptors with maturity, we characterized the specificity of NVP-AAM007. We found not only that NVP-AAM007 lacks discernable subunit specificity but also that the effects of NVP-AAM077 on LTP could be mimicked using subsaturating concentrations of APV, a global NMDAR antagonist. These results indicate that the effects of NVP-AAM077 on synaptic plasticity are largely explained by nonspecific blockade of NMDARs. Moreover our findings are the first to reveal a developmental increase in the sensitivity of LTP to NMDAR antagonism. We suggest that discrepant reports describing the effect of NVP-AAM077 on LTP may be partially explained by this developmental shift in the properties of LTP. These results indicate that the degree of NMDAR activation required for LTP increases with development, providing insight into a novel underlying mechanism governing the properties of synaptic plasticity.
Figures





References
-
- Auberson YP, Allgeier H, Bischoff S, Lingenhoehl K, Moretti R, Schmutz M. 5-Phosphonomethylquinoxalinediones as competitive NMDA receptor antagonists with a preference for the human 1A/2A, rather than 1A/2B receptor composition. Bioorg Med Chem Lett 12: 1099–1102, 2002. - PubMed
-
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron 48: 289–301, 2005. - PubMed
-
- Bartlett TE, Bannister NJ, Collett VJ, Dargan SL, Massey PV, Bortolotto ZA, Fitzjohn SM, Bashir ZI, Collingridge GL, Lodge D. Differential roles of NR2A and NR2B-containing NMDA receptors in LTP and LTD in the CA1 region of two-week old rat hippocampus. Neuropharmacology 52: 60–70, 2007. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

