Analog modulation of mossy fiber transmission is uncoupled from changes in presynaptic Ca2+
- PMID: 18667608
- PMCID: PMC2685171
- DOI: 10.1523/JNEUROSCI.1296-08.2008
Analog modulation of mossy fiber transmission is uncoupled from changes in presynaptic Ca2+
Abstract
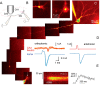
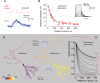
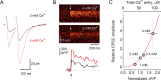
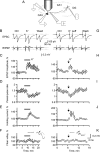
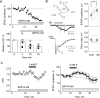
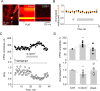
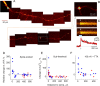
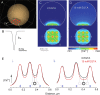
Subthreshold somatic depolarization has been shown recently to modulate presynaptic neurotransmitter release in cortical neurons. To understand the mechanisms underlying this mode of signaling in the axons of dentate granule cells (hippocampal mossy fibers), we have combined two-photon Ca2+ imaging with dual-patch recordings from somata and giant boutons forming synapses on CA3 pyramidal cells. In intact axons, subthreshold depolarization propagates both orthodromically and antidromically, with an estimated length constant of 200-600 microm depending on the signal waveform. Surprisingly, presynaptic depolarization sufficient to enhance glutamate release at mossy fiber-CA3 pyramidal cell synapses has no detectable effect on either basal Ca2+-dependent fluorescence or action-potential-evoked fluorescence transients in giant boutons. We further estimate that neurotransmitter release varies with presynaptic Ca2+ entry with a 2.5-power relationship and that depolarization-induced synaptic facilitation remains intact in the presence of high-affinity presynaptic Ca2+ buffers or after blockade of local Ca2+ stores. We conclude that depolarization-dependent modulation of transmission at these boutons does not rely on changes in presynaptic Ca2+.
Figures

References
-
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. - PubMed
-
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
