Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina
- PMID: 18667624
- PMCID: PMC2575372
- DOI: 10.1523/JNEUROSCI.0784-08.2008
Diverse mechanisms underlie glycinergic feedback transmission onto rod bipolar cells in rat retina
Abstract
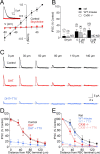
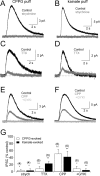
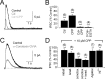
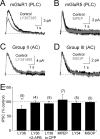
Synaptic inhibition shapes visual signaling in the inner retina, but the physiology of most amacrine cells, the interneurons that mediate this inhibition, is poorly understood. Discerning the function of most individual amacrine cell types is a daunting task, because few molecular or morphological markers specifically distinguish between approximately two dozen different amacrine cell types. Here, we examine a functional subset of amacrine cells by pharmacologically isolating glycinergic inhibition and evoking feedback IPSCs in a single cell type, the rod bipolar cell (RBC), with brief glutamate applications in the inner plexiform layer. We find that glycinergic amacrine cells innervating RBCs receive excitatory inputs from ON and OFF bipolar cells primarily via NMDA receptors (NMDARs) and Ca2+-impermeable AMPA-type glutamate receptors. Glycine release from amacrine cells is triggered by Ca2+ influx through both voltage-gated Ca2+ (Ca(v)) channels and NMDARs. These intracellular Ca2+signals are amplified by Ca2+-induced Ca2+ release via both ryanodine and IP3 receptors, which are activated independently by Ca2+ influx through Ca(v) channels and NMDARs, respectively. Glycinergic feedback signaling depends strongly, although not completely, on voltage-gated Na+ channels, and the spatial extent of feedback inhibition is expanded by gap junction connections between glycinergic amacrine cells. These results indicate that a diversity of mechanisms underlie glycinergic feedback inhibition onto RBCs, yet they highlight several physiological themes that appear to distinguish amacrine cell function.
Figures




References
-
- Berridge MJ. Neuronal calcium signaling. Neuron. 1998;21:13–26. - PubMed
-
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
-
- Bieda MC, Copenhagen DR. Sodium action potentials are not required for light-evoked release of GABA or glycine from retinal amacrine cells. J Neurophysiol. 1999;81:3092–3095. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
