IL-33 exacerbates antigen-induced arthritis by activating mast cells
- PMID: 18667700
- PMCID: PMC2491487
- DOI: 10.1073/pnas.0801898105
IL-33 exacerbates antigen-induced arthritis by activating mast cells
Abstract
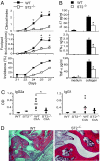
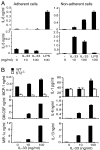
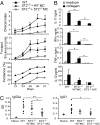
IL-33, a cytokine of the IL-1 family, is closely associated with type II T cell responses. Here, we report an unexpected proinflammatory role of IL-33 in inflammatory arthritis. IL-33 was expressed in synovial fibroblasts from patients with rheumatoid arthritis (RA). Expression was markedly elevated in vitro by inflammatory cytokines. Mice lacking ST2, the IL-33 receptor alpha-chain, developed attenuated collagen-induced arthritis (CIA) and reduced ex vivo collagen-specific induction of proinflammatory cytokines (IL-17, TNFalpha, and IFNgamma), and antibody production. Conversely, treatment of wild-type (WT) but not ST2(-/-) mice with IL-33 exacerbated CIA and elevated production of both proinflammatory cytokines and anti-collagen antibodies. Mast cells expressed high levels of ST2 and responded directly to IL-33 to produce a spectrum of inflammatory cytokines and chemokines in vitro. In vivo, IL-33 treatment exacerbated CIA in ST2(-/-) mice engrafted with mast cells from WT but not from ST2(-/-) mice. Disease exacerbation was accompanied by elevated expression levels of proinflammatory cytokines. Our results demonstrate that IL-33 is a critical proinflammatory cytokine for inflammatory joint disease that integrates fibroblast activation with downstream immune activation mainly via an IL-33-driven, mast-cell-dependent pathway. Thus, this IL-1 superfamily member represents a therapeutic target for RA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Schmitz JA, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. - PubMed
-
- Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20:S1–13. - PubMed
-
- Chackerian AA, et al. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

