Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats
- PMID: 18667718
- PMCID: PMC2576087
- DOI: 10.1152/ajpregu.90309.2008
Intermittent access to preferred food reduces the reinforcing efficacy of chow in rats
Abstract
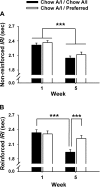
Intermittent, extended access to preferred diets increases their intake. However, the effects of such access on the acceptance and reinforcing efficacy of otherwise satisfying alternatives is less known. To investigate the role of nonnutritional contributions to the hypophagia that follows removal of preferred food, male Wistar rats were fed a chow diet (Chow A/I), preferred to their regular chow (Chow), which was equally consumed under 1-choice conditions to an even more preferred chocolate-flavored, sucrose-rich diet (Preferred). Rats then learned to obtain Chow A/I pellets under a progressive ratio schedule of reinforcement and were assigned to two matched groups. Each week, one group (n = 15) was diet-cycled, receiving Chow A/I for 5 days followed by the Preferred diet for 2 days. Controls received Chow A/I daily (n = 14). Progressive ratio sessions were performed daily during the 5 days that all subjects received Chow A/I in the home cage. Across 5 wk, diet-cycled rats progressively ate less of the otherwise palatable Chow A/I diet. Hypophagia was not due to greater prior intake or weight gain, motor impairment, or facilitated satiation and was associated with changes in progressive ratio performance that suggested a reduced reinforcing efficacy of the Chow A/I diet in diet-cycled animals. By week 4, diet-cycled animals began to overeat the preferred diet, especially during the first 6 h of renewed access, resembling a deprivation effect. The results suggest that intermittent access to highly preferred food, as practiced by many restrained eaters, may progressively decrease the acceptability of less palatable foods, and may promote relapse to more rewarding alternatives.
Figures




Similar articles
-
Consummatory, anxiety-related and metabolic adaptations in female rats with alternating access to preferred food.Psychoneuroendocrinology. 2009 Jan;34(1):38-49. doi: 10.1016/j.psyneuen.2008.08.010. Epub 2008 Oct 8. Psychoneuroendocrinology. 2009. PMID: 18842344 Free PMC article.
-
The duration of intermittent access to preferred sucrose-rich food affects binge-like intake, fat accumulation, and fasting glucose in male rats.Appetite. 2018 Nov 1;130:59-69. doi: 10.1016/j.appet.2018.07.025. Epub 2018 Jul 29. Appetite. 2018. PMID: 30063959 Free PMC article.
-
Intermittent, extended access to preferred food leads to escalated food reinforcement and cyclic whole-body metabolism in rats: Sex differences and individual vulnerability.Physiol Behav. 2018 Aug 1;192:3-16. doi: 10.1016/j.physbeh.2018.04.001. Epub 2018 Apr 11. Physiol Behav. 2018. PMID: 29654812 Free PMC article.
-
Opioid-dependent anticipatory negative contrast and binge-like eating in rats with limited access to highly preferred food.Neuropsychopharmacology. 2008 Feb;33(3):524-35. doi: 10.1038/sj.npp.1301430. Epub 2007 Apr 18. Neuropsychopharmacology. 2008. PMID: 17443124
-
Food reinforcement and eating: a multilevel analysis.Psychol Bull. 2007 Sep;133(5):884-906. doi: 10.1037/0033-2909.133.5.884. Psychol Bull. 2007. PMID: 17723034 Free PMC article. Review.
Cited by
-
High trait impulsivity predicts food addiction-like behavior in the rat.Neuropsychopharmacology. 2014 Sep;39(10):2463-72. doi: 10.1038/npp.2014.98. Epub 2014 Apr 29. Neuropsychopharmacology. 2014. PMID: 24776685 Free PMC article.
-
Antagonism of sigma-1 receptors blocks compulsive-like eating.Neuropsychopharmacology. 2012 Nov;37(12):2593-604. doi: 10.1038/npp.2012.89. Epub 2012 Jun 20. Neuropsychopharmacology. 2012. PMID: 22713906 Free PMC article.
-
Removal of high-fat diet after chronic exposure drives binge behavior and dopaminergic dysregulation in female mice.Neuroscience. 2016 Jun 21;326:170-179. doi: 10.1016/j.neuroscience.2016.04.002. Epub 2016 Apr 8. Neuroscience. 2016. PMID: 27063418 Free PMC article.
-
Increased ethanol consumption after interruption of fat bingeing.PLoS One. 2018 Mar 28;13(3):e0194431. doi: 10.1371/journal.pone.0194431. eCollection 2018. PLoS One. 2018. PMID: 29590149 Free PMC article.
-
Reward sensitivity deficits in a rat model of compulsive eating behavior.Neuropsychopharmacology. 2020 Mar;45(4):589-596. doi: 10.1038/s41386-019-0550-1. Epub 2019 Oct 17. Neuropsychopharmacology. 2020. PMID: 31622973 Free PMC article.
References
-
- Ahmed SH, Kenny PJ, Koob GF, Markou A. Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5: 625–626, 2002. - PubMed
-
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set point. Science 282: 298–300, 1998. - PubMed
-
- Ahmed SH, Walker JR, Koob GF. Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology 22: 413–421, 2000. - PubMed
-
- Archer ZA, Rayner DV, Barrett P, Balik A, Duncan JS, Moar KM, Mercer JG. Hypothalamic energy balance gene responses in the Sprague-Dawley rat to supplementation of high-energy diet with liquid ensure and subsequent transfer to chow. J Neuroendocrinol 17: 711–719, 2005. - PubMed
-
- Aujla H, Martin-Fardon R, Weiss F. Rats with extended access to cocaine exhibit increased stress reactivity and sensitivity to the anxiolytic-like effects of the mGluR 2/3 agonist LY379268 during abstinence. Neuropsychopharmacology 33: 1818–1826, 2007. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

