Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area
- PMID: 18667749
- PMCID: PMC2714996
- DOI: 10.1095/biolreprod.108.069831
Restoration of the luteinizing hormone surge in middle-aged female rats by altering the balance of GABA and glutamate transmission in the medial preoptic area
Abstract
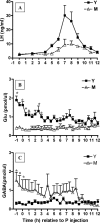
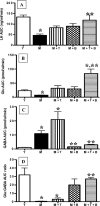
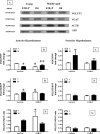
Hypothalamic glutamate and gamma-aminobutyric acid (GABA) neurotransmission are involved in the ovarian hormone-induced GnRH-LH surge in rodents. We previously reported that middle-aged rats have significantly less glutamate release in the medial preoptic area than young rats on the day of the LH surge. The present study tested the hypothesis that the delayed and attenuated LH surge in ovariohysterectomized middle-aged rats primed with ovarian steroids results from reduced hypothalamic glutamate and increased GABA(A) neurotransmission. Microdialysis results show that middle-aged rats with attenuated LH surges had reduced extracellular glutamate and increased extracellular GABA levels in the medial preoptic area compared with young rats. Blocking GABA(A) receptors with bicuculline or inhibiting synaptic glutamate reuptake with L-trans-pyrrolidine-2,4-dicarboxylic acid increased extracellular Glu in the medial preoptic area and partially restored LH surge amplitude in middle-aged rats without altering LH surge onset. Complete recovery of LH surge amplitude was observed in middle-aged rats treated with the combination of bicuculline and L-trans-pyrrolidine-2,4-dicarboxylic acid. This treatment also restored the extracellular glutamate:GABA ratio in the medial preoptic area of middle-aged rats to the level of young rats. Immunoblot analysis revealed that estradiol and progesterone treatment reduced SLC32A1(formerly known as vesicular GABA transporter) levels and increased SLC17A6 (formerly known as vesicular glutamate transporter 2) levels in the anterior hypothalamus of ovariohysterectomized young but not middle-aged rats. These data suggest that both reduced availability of glutamate and increased activation of GABA(A) receptors under estrogen-positive feedback conditions contribute to the age-related delay in onset and attenuated amplitude of the LH surge.
Figures


References
-
- Burger HG, Dudley EC, Robertson DM, Dennerstein L.Hormonal changes in the menopause transition. Recent Prog Horm Res 2002; 57: 257–275.. - PubMed
-
- Park SJ, Goldsmith LT, Weiss G.Age-related changes in the regulation of luteinizing hormone secretion by estrogen in women. Exp Biol Med 2002; 227: 455–464.. - PubMed
-
- Finch CE, Felicio LS, Mobbs CV, Nelson JF.Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. Endocr Rev 1984; 5: 467–497.. - PubMed
-
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF.Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum Reprod 1992; 7: 1342–1346.. - PubMed
-
- Burger HG, Dudley EC, Hopper JL, Shelley JM, Green A, Smith A, Dennerstein L, Morse C.The endocrinology of the menopausal transition: a cross-sectional study of a population-based sample. J Clin Endocrinol Metab 1995; 80: 3537–3545.. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

