Bioluminescent virion shells: new tools for quantitation of AAV vector dynamics in cells and live animals
- PMID: 18668144
- PMCID: PMC2684674
- DOI: 10.1038/gt.2008.127
Bioluminescent virion shells: new tools for quantitation of AAV vector dynamics in cells and live animals
Abstract
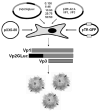
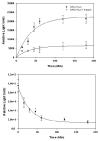
Current technologies for visualizing infectious pathways of viruses rely on fluorescent labeling of capsid proteins by chemical conjugation or genetic manipulation. For noninvasive in vivo imaging of such agents in mammalian tissue, we engineered bioluminescent Gaussia luciferase-tagged Adeno-associated viral (gLuc/AAV) vectors. The enzyme was incorporated into recombinant AAV serotypes 1, 2 and 8 capsids by fusing to the N-terminus of the VP2 capsid subunit to yield bioluminescent virion shells. The gLuc/AAV vectors were used to quantify kinetics of cell-surface-binding by AAV2 capsids in vitro. Bioluminescent virion shells displayed an exponential decrease in luminescent signal following cellular uptake in vitro. A similar trend was observed following intramuscular injection in vivo, although the rate of decline in bioluminescent signal varied markedly between AAV serotypes. gLuc/AAV1 and gLuc/AAV8 vectors displayed rapid decrease in bioluminescent signal to background levels within 30 min, whereas the signal from gLuc/AAV2 vectors persisted for over 2 h. Bioluminescent virion shells might be particularly useful in quantifying dynamics of viral vector uptake in cells and peripheral tissues in live animals.
Figures


References
-
- Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14:316–327. - PubMed
-
- Swenson ES, Price JG, Brazelton T, Krause DS. Limitations of green fluorescent protein as a cell lineage marker. Stem Cells. 2007;25:2593–2600. - PubMed
-
- Tam JM, Upadhyay R, Pittet MJ, Weissleder R, Mahmood U. Improved in vivo whole-animal detection limits of green fluorescent protein-expressing tumor lines by spectral fluorescence imaging. Mol Imaging. 2007;6:269–276. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

