Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma
- PMID: 18669462
- PMCID: PMC2504739
- DOI: 10.1200/JCO.2007.14.3974
Amifostine protects against cisplatin-induced ototoxicity in children with average-risk medulloblastoma
Abstract
Purpose: To determine the role of amifostine as a protectant against cisplatin-induced ototoxicity in patients with average-risk (AR) medulloblastoma treated with craniospinal radiotherapy and four cycles of cisplatin-based, dose-intense chemotherapy and stem-cell rescue.
Patients and methods: The primary objective was to determine whether, in patients with AR medulloblastoma (n = 62), amifostine would decrease the need for hearing aids (defined as >or= grade 3 ototoxicity in one ear) compared with a control group (n = 35), 1 year from initiating treatment. Ninety-seven patients received craniospinal irradiation (23.4 Gy) followed by 55.8 Gy to the primary tumor bed using three-dimensional conformal technique, and four cycles of high-dose cyclophosphamide (4,000 mg/m(2)/cycle), cisplatin (75 mg/m(2)/cycle), and vincristine (two 1.5 mg/m(2) doses/cycle) and stem-cell rescue. When used, amifostine (600 mg/m(2)/dose) was administered as a bolus immediately before and 3 hours into the cisplatin infusion.
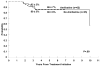
Results: The median age of the 97 patients was 8.7 years (range, 3.2 to 20.2 years). The study and control groups were similar in age and sex distribution. Amifostine was well-tolerated. One year after treatment initiation, 13 patients (37.1%) in the control group versus nine (14.5%; one-sided chi(2) test P = .005) of the amifostine-treated patients had at least grade 3 ototoxicity, requiring hearing aid in at least one ear.
Conclusion: Amifostine administered before and during the cisplatin infusion can significantly reduce the risk of severe ototoxicity in patients with AR medulloblastoma receiving dose-intense chemotherapy.
Figures
Comment in
-
Prevention of hearing loss in children receiving cisplatin chemotherapy.J Clin Oncol. 2009 Jan 10;27(2):317-8; author reply 318-9. doi: 10.1200/JCO.2008.20.1160. Epub 2008 Dec 8. J Clin Oncol. 2009. PMID: 19064953 No abstract available.
References
-
- Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7:813–820. - PubMed
-
- Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24:4202–4208. - PubMed
-
- Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children’s Cancer Group Study. J Clin Oncol. 1999;17:2127–2136. - PubMed
-
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23:8588–8596. - PubMed
-
- Kemp G, Rose P, Lurain J, et al. Amifostine pretreatment for protection against cyclophosphamide-induced and cisplatin-induced toxicities: results of a randomized control trial in patients with advanced ovarian cancer. J Clin Oncol. 1996;14:2101–2112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials