Blinded independent central review of progression-free survival in phase III clinical trials: important design element or unnecessary expense?
- PMID: 18669467
- PMCID: PMC2654812
- DOI: 10.1200/JCO.2008.16.1711
Blinded independent central review of progression-free survival in phase III clinical trials: important design element or unnecessary expense?
Erratum in
- J Clin Oncol. 2009 Apr 20;27(12):2109-10
Abstract
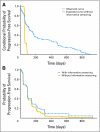
Progression-free survival is an important end point in advanced disease settings. Blinded independent central review (BICR) of progression in randomized clinical trials has been advocated to control bias that might result from errors in progression assessments. However, although BICR lessens some potential biases, it does not remove all biases from evaluations of treatment effectiveness. In fact, as typically conducted, BICRs may introduce bias because of informative censoring, which results from having to censor unconfirmed locally determined progressions. In this article, we discuss the rationale for BICR and different ways of implementing independent review. We discuss the limitations of these approaches and review published trials that report implementing BICR. We demonstrate the existence of informative censoring using data from a randomized phase II trial. We conclude that double-blinded trials with consistent application of measurement criteria are the best means of ensuring unbiased trial results. When such designs are not practical, BICR is not recommended as a general strategy for reducing bias. However, BICR may be useful as an auditing tool to assess the reliability of marginally positive results.
Figures
Comment in
-
Onsite image evaluations and independent image blinded reads: close cousins or distant relatives?J Clin Oncol. 2009 Apr 20;27(12):2103-4; author reply 2104-5. doi: 10.1200/JCO.2008.21.3447. Epub 2009 Mar 9. J Clin Oncol. 2009. PMID: 19273694 No abstract available.
-
Are onsite image evaluations the solution or are we trading one problem for another?J Clin Oncol. 2009 Dec 10;27(35):e263-4; author reply e265. doi: 10.1200/JCO.2009.25.3716. Epub 2009 Nov 9. J Clin Oncol. 2009. PMID: 19901131 No abstract available.
References
-
- Johnson JR, Williams G, Pazdur R: End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol 21:1404-1411, 2003 - PubMed
-
- Freidlin B, Korn EL, Hunsberger S, et al: Proposal for the use of progression-free survival in unblinded randomized trials. J Clin Oncol 25:2122-2126, 2007 - PubMed
-
- Therasse P, Arbuck SG, Eisenhauer EA, et al: New guidelines to evaluate the response to treatment in solid tumors: European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92:205-216, 2000 - PubMed
-
- Miller AB, Hoogstraten B, Staguet M, et al: Reporting results of cancer treatment. Cancer 47:207-214, 1981 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

