Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight
- PMID: 18669597
- PMCID: PMC2584592
- DOI: 10.1210/en.2008-0805
Selective inactivation of Socs3 in SF1 neurons improves glucose homeostasis without affecting body weight
Abstract
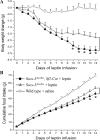
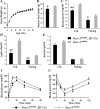
Suppressor of cytokine signaling 3 (Socs3) has been identified as a mediator of central leptin resistance, but the identity of specific neurons in which Socs3 acts to suppress leptin signaling remains elusive. The ventromedial hypothalamus (VMH) was recently shown to be an important site for leptin action because deleting leptin receptor within VMH neurons causes obesity. To examine the role of VMH Socs3 in leptin resistance and energy homeostasis, we generated mice lacking Socs3 specifically in neurons positive for steroidogenic factor 1 (SF1), which is expressed abundantly in the VMH. These mice had increased phosphorylation of signal transducer and activator of transcription-3 in VMH neurons, suggesting improved leptin signaling, and consistently, food intake and weight-reducing effects of exogenous leptin were enhanced. Furthermore, on either chow or high-fat diets, these mice had reduced food intake. Unexpectedly, energy expenditure was reduced as well. Mice lacking Socs3 in SF1 neurons, despite no change in body weight, had improved glucose homeostasis and were partially protected from hyperglycemia and hyperinsulinemia induced by high-fat diets. These results suggest that Socs3 in SF1 neurons negatively regulates leptin signaling and plays important roles in mediating leptin sensitivity, glucose homeostasis, and energy expenditure.
Figures



References
-
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 - PubMed
-
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM 1995 Weight-reducing effects of the plasma protein encoded by the obese gene. Science 269:543–546 - PubMed
-
- Pelleymounter MA, Cullen MJ, Baker MB, Hecht R, Winters D, Boone T, Collins F 1995 Effects of the obese gene product on body weight regulation in ob/ob mice. Science 269:540–543 - PubMed
-
- Campfield LA, Smith FJ, Guisez Y, Devos R, Burn P 1995 Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science 269:546–549 - PubMed
-
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O'Rahilly S 1999 Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med 341:879–884 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

