FE(II) is the native cofactor for Escherichia coli methionine aminopeptidase
- PMID: 18669631
- PMCID: PMC2556001
- DOI: 10.1074/jbc.M804345200
FE(II) is the native cofactor for Escherichia coli methionine aminopeptidase
Abstract
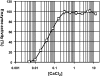
Divalent metal ions play a critical role in the removal of N-terminal methionine from nascent proteins by methionine aminopeptidase (MetAP). Being an essential enzyme for bacteria, MetAP is an appealing target for the development of novel antibacterial drugs. Although purified enzyme can be activated by several divalent metal ions, the exact metal ion used by MetAP in cells is unknown. Many MetAP inhibitors are highly potent on purified enzyme, but they fail to show significant inhibition of bacterial growth. One possibility for the failure is a disparity of the metal used in activation of purified MetAP and the metal actually used by MetAP inside bacterial cells. Therefore, the challenge is to elucidate the physiologically relevant metal for MetAP and discover MetAP inhibitors that can effectively inhibit cellular MetAP. We have recently discovered MetAP inhibitors with selectivity toward different metalloforms of Escherichia coli MetAP, and with these unique inhibitors, we characterized their inhibition of MetAP enzyme activity in a cellular environment. We observed that only inhibitors that are selective for the Fe(II)-form of MetAP were potent in this assay. Further, we found that only these Fe(II)-form selective inhibitors showed significant inhibition of growth of five E. coli strains and two Bacillus strains. We confirmed their cellular target as MetAP by analysis of N-terminal processed and unprocessed recombinant glutathione S-transferase proteins. Therefore, we conclude that Fe(II) is the likely metal used by MetAP in E. coli and other bacterial cells.
Figures
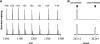
References
-
- Bradshaw, R. A., Brickey, W. W., and Walker, K. W. (1998) Trends Biochem. Sci. 23 263–267 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

