GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein
- PMID: 18669654
- PMCID: PMC2504800
- DOI: 10.1073/pnas.0802437105
GATA transcription factors directly regulate the Parkinson's disease-linked gene alpha-synuclein
Abstract
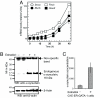
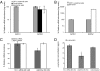
Increased alpha-synuclein gene (SNCA) dosage due to locus multiplication causes autosomal dominant Parkinson's disease (PD). Variation in SNCA expression may be critical in common, genetically complex PD but the underlying regulatory mechanism is unknown. We show that SNCA and the heme metabolism genes ALAS2, FECH, and BLVRB form a block of tightly correlated gene expression in 113 samples of human blood, where SNCA naturally abounds (validated P = 1.6 x 10(-11), 1.8 x 10(-10), and 6.6 x 10(-5)). Genetic complementation analysis revealed that these four genes are co-induced by the transcription factor GATA-1. GATA-1 specifically occupies a conserved region within SNCA intron-1 and directly induces a 6.9-fold increase in alpha-synuclein. Endogenous GATA-2 is highly expressed in substantia nigra vulnerable to PD, occupies intron-1, and modulates SNCA expression in dopaminergic cells. This critical link between GATA factors and SNCA may enable therapies designed to lower alpha-synuclein production.
Conflict of interest statement
Conflict of interest statement: C.R.S., E.H.B., and M.G.S. are listed as coinventors on a United States patent application related to the development of therapeutics for Parkinson's disease.
Figures

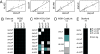

References
-
- Singleton AB, et al. α-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302:841. - PubMed
-
- Maraganore DM, et al. Collaborative analysis of α-synuclein gene promoter variability and Parkinson disease. J Am Med Assoc. 2006;296:661–670. - PubMed
-
- Farrer M, et al. Comparison of kindreds with parkinsonism and α-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. - PubMed
-
- Scherzer CR, Feany MB. Yeast genetics targets lipids in Parkinson's disease. Trends Genet. 2004;20:273–277. - PubMed
-
- Klein C, Schlossmacher MG. The genetics of Parkinson disease: Implications for neurological care. Nat Clin Pract Neurol. 2006;2:136–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

