Duration of intrapartum prophylaxis and concentration of penicillin G in fetal serum at delivery
- PMID: 18669721
- PMCID: PMC3144749
- DOI: 10.1097/AOG.0b013e31817d0246
Duration of intrapartum prophylaxis and concentration of penicillin G in fetal serum at delivery
Abstract
Objective: Intrapartum penicillin G prophylaxis aims to prevent early-onset group B streptococci (GBS) sepsis by interrupting vertical transmission. We examined the relationship between duration of prophylaxis and fetal serum penicillin G levels among fetuses exposed to fewer than 4 hours of prophylaxis compared with longer durations.
Methods: In this prospective cohort study, 98 laboring GBS-positive women carrying singleton gestations at 37 weeks or greater were administered 5 million units of intravenous penicillin G followed by 2.5 million units every 4 hours until delivery. Umbilical cord blood samples were collected at delivery, and penicillin G levels were measured by high-performance liquid chromatography. Intraassay and interassay coefficients of variation were less than 3%.
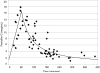
Results: Fetuses exposed to fewer than 4 hours of prophylaxis had higher penicillin G levels than those exposed to greater than 4 hours (P=.003). In multivariable linear regression analysis, fetal penicillin G levels were determined by duration of exposure, time since last dose, dosage, and number of doses, but not maternal body mass index. Penicillin G levels increased linearly until 1 hour (R(2)=.40) and then decreased rapidly according to a power-decay model (R(2)=.67). All subgroups analyzed were above the minimal inhibitory concentration (MIC) for GBS (0.1 micrograms/mL)(P<.002). Individual samples were 10-179-fold above the MIC. In patients receiving maintenance dosing, penicillin G did not accumulate in the cord blood and returned to baseline after each 4-hour interval.
Conclusion: Short durations of prophylaxis achieved levels significantly above the MIC, suggesting a benefit even in precipitous labors. The designation of infants exposed to fewer than 4 hours of prophylaxis as particularly at risk for GBS sepsis may be pharmacokinetically inaccurate.
Figures

References
-
-
Schuchat A. Epidemiology of group B streptococcal disease in the United States: shifting paradigms. Clin Microbiol Rev. 1998;11:497–513. Author: Journal style calls for six author names before the use of “et al.” I filled in missing author names from MEDLINE entries. Please check to see that this was done accurately. Where necessary I corrected references to match the MEDLINE listings.
-
-
- Gibbs RS, Schrag S, Schuchat A. Perinatal infections due to group B streptococci. Obstet Gynecol. 2004;104:1062–76. - PubMed
-
- Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of perinatal group B streptococcal disease. Revised guidelines from CDC. MMWR Recomm Rep. 2002;51:1–22. - PubMed
-
- Boyer KM, Gotoff SP. Prevention of early-onset neonatal group B streptococcal disease with selective intrapartum chemoprophylaxis. N Engl J Med. 1986;314:1665–9. - PubMed
-
- Lin FY, Brenner RA, Johnson YR, Azimi PH, Philips JB, 3rd, Regan JA. The effectiveness of risk-based intrapartum chemoprophylaxis for the prevention of early-onset neonatal group B streptococcal disease. Am J Obstet Gynecol. 2001;184:1204–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

