Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke
- PMID: 18669895
- PMCID: PMC2740650
- DOI: 10.1161/STROKEAHA.108.515684
Synergistic effect of an endothelin type A receptor antagonist, S-0139, with rtPA on the neuroprotection after embolic stroke
Abstract
Background and purpose: Using a model of embolic stroke, the present study tested the hypothesis that blockage of endothelin-1 with S-0139, a specific endothelin type A receptor (ET(A)) antagonist, enhances the neuroprotective effect of recombinant tissue plasminogen activator (rtPA) by suppressing molecules that mediate thrombosis and blood brain barrier (BBB) disruption induced by ischemia and rtPA.
Methods: Rats (n=104) subjected to embolic middle cerebral artery (MCA) occlusion were randomly divided into 1 of 4 infusion groups with 26 rats per group: (1) the control group in which rats were administered saline, (2) the monotherapy rtPA group in which rtPA was intravenously administered at a dose of 10 mg/kg 4 hours after MCA occlusion, (3) the monotherapy S-0139 group in which S-0139 was intravenously given 2 hours after MCA occlusion, and (4) the combination of rtPA +S-0139 group in which S-0139 and rtPA were given 2 and 4 hours after MCA occlusion, respectively. Measurements of infarct volume and parenchymal hemorrhage, behavioral outcome, and immunostaining were performed on rats euthanized 1 and 7 days after stroke.
Results: The combination therapy of S-0139 and rtPA significantly (P<0.01) reduced infarct volume (24.8+/-0.9% versus 33.8+/-1.5% in control) and hemorrhagic area (7.1+/-6.1 microm(2) versus 36.5+/-19.2 microm(2) in control) and improved functional recovery compared with control saline-treated animals. Immunostaining analysis revealed that the combination therapy had the synergistically suppressed ischemia- and rtPA-induced ICAM-1, protease-activated receptor 1 (PAR-1), as well as accumulation of platelets in cerebral microvessels. Furthermore, the combination treatment synergistically reduced loss of laminin, ZO1, and occludin in cerebral vessels.
Conclusions: These data suggest that S-0139 provides the neuroprotection by suppressing ischemia- and rtPA-triggered molecules that evoke thrombosis and BBB disruption.
Figures



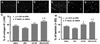
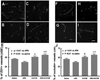
References
-
- Stanimirovic DB, Yamamoto T, Uematsu S, Spatz M. Endothelin-1 receptor binding and cellular signal transduction in cultured human brain endothelial cells. J Neurochem. 1994;62:592–601. - PubMed
-
- Narushima I, Kita T, Kubo K, Yonetani Y, Momochi C, Yoshikawa I, Ohno N, Nakashima T. Highly enhanced permeability of blood-brain barrier induced by repeated administration of endothelin-1 in dogs and rats. Pharmacol Toxicol. 2003;92:21–26. - PubMed
-
- Matsuo Y, Mihara S, Ninomiya M, Fujimoto M. Protective effect of endothelin type a receptor antagonist on brain edema and injury after transient middle cerebral artery occlusion in rats. Stroke. 2001;32:2143–2148. - PubMed
-
- Volpe M, Cosentino F. Abnormalities of endothelial function in the pathogenesis of stroke: The importance of endothelin. J Cardiovasc Pharmacol. 2000;35:S45–S48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

