In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish
- PMID: 18670586
- PMCID: PMC2186061
- DOI: 10.1016/j.carbon.2007.04.021
In vivo evaluation of carbon fullerene toxicity using embryonic zebrafish
Abstract
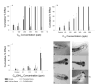
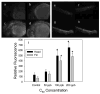
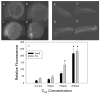
There is a pressing need to develop rapid whole animal-based testing assays to assess the potential toxicity of engineered nanomaterials. To meet this challenge, the embryonic zebrafish model was employed to determine the toxicity of fullerenes. Embryonic zebrafish were exposed to graded concentrations of fullerenes [C(60), C(70), and C(60)(OH)(24)] during early embryogenesis and the resulting morphological and cellular responses were defined. Exposure to 200 μg/L C(60) and C(70) induced a significant increased in malformations, pericardial edema, and mortality; while the response to C(60)(OH)(24) exposure was less pronounced at concentrations an order of magnitude higher. Exposure to C(60) induced both necrotic and apoptotic cellular death throughout the embryo. While C(60)(OH)(24) induced an increase in embryonic cellular death, it did not induce apoptosis. Our findings concur with results obtained in other models indicating that C(60)(OH)(24) is significantly less toxic than C(60). These studies also suggest that that the embryonic zebrafish model is well-suited for the rapid assessment of nanomaterial toxicity.
Figures

References
-
- Lecoanet HF, Bottero JY, Wiesner MR. Laboratory assessment of the mobility of nanomaterials in porous media. Environmental Science & Technology. 2004;38(19):5164–5169. - PubMed
-
- Lecoanet HF, Wiesner MR. Velocity effects on fullerene and oxide nanoparticle deposition in porous media. Environmental Science & Technology. 2004;38(16):4377–4382. - PubMed
-
- Lecoanet H, Wiesner MR. Assessment of the mobility of nanomaterials in groundwater acouifers. Abstracts of Papers of the American Chemical Society. 2004;227:U1275–U1275.
-
- Okamoto Y. Ab initio investigation of hydrogenation of C-60. Journal of Physical Chemistry A. 2001;105(32):7634–7637.
-
- Sun O, Wang Q, Jena P, Kawazoe Y. Clustering of Ti on a C-60 surface and its effect on hydrogen storage. Journal of the American Chemical Society. 2005;127(42):14582–14583. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
