Efficient genetic method for establishing Drosophila cell lines unlocks the potential to create lines of specific genotypes
- PMID: 18670627
- PMCID: PMC2474701
- DOI: 10.1371/journal.pgen.1000142
Efficient genetic method for establishing Drosophila cell lines unlocks the potential to create lines of specific genotypes
Abstract
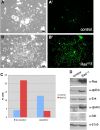
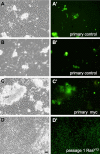
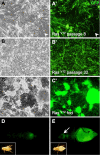
Analysis of cells in culture has made substantial contributions to biological research. The versatility and scale of in vitro manipulation and new applications such as high-throughput gene silencing screens ensure the continued importance of cell-culture studies. In comparison to mammalian systems, Drosophila cell culture is underdeveloped, primarily because there is no general genetic method for deriving new cell lines. Here we found expression of the conserved oncogene Ras(V12) (a constitutively activated form of Ras) profoundly influences the development of primary cultures derived from embryos. The cultures become confluent in about three weeks and can be passaged with great success. The lines have undergone more than 90 population doublings and therefore constitute continuous cell lines. Most lines are composed of spindle-shaped cells of mesodermal type. We tested the use of the method for deriving Drosophila cell lines of a specific genotype by establishing cultures from embryos in which the warts (wts) tumor suppressor gene was targeted. We successfully created several cell lines and found that these differ from controls because they are primarily polyploid. This phenotype likely reflects the known role for the mammalian wts counterparts in the tetraploidy checkpoint. We conclude that expression of Ras(V12) is a powerful genetic mechanism to promote proliferation in Drosophila primary culture cells and serves as an efficient means to generate continuous cell lines of a given genotype.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Eschalier G. Drosophila Cells in Culture. New York: Academic Press; 1997.
-
- Simcox AA, Sobeih MM, Shearn A. Establishment and characterization of continuous cell lines derived from temperature-sensitive mutants of Drosophila melanogaster. Somatic Cell and Molecular Genetics. 1985;11:63–70. - PubMed
-
- Schneider I. Cell lines derived from late embryonic stages of Drosophila melanogaster. J Embryol Exp Morphol. 1972;27:353–365. - PubMed
-
- Debec A. Haploid cell cultures of Drosophila melanogaster. Nature. 1978;274:255–256. - PubMed
-
- Echalier G, Ohanessian A. [Isolation, in tissue culture, of Drosophila melangaster cell lines]. C R Acad Sci Hebd Seances Acad Sci D. 1969;268:1771–1773. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

