H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice
- PMID: 18670648
- PMCID: PMC2483250
- DOI: 10.1371/journal.ppat.1000115
H5N1 and 1918 pandemic influenza virus infection results in early and excessive infiltration of macrophages and neutrophils in the lungs of mice
Abstract
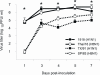
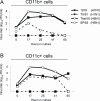
Fatal human respiratory disease associated with the 1918 pandemic influenza virus and potentially pandemic H5N1 viruses is characterized by severe lung pathology, including pulmonary edema and extensive inflammatory infiltrate. Here, we quantified the cellular immune response to infection in the mouse lung by flow cytometry and demonstrate that mice infected with highly pathogenic (HP) H1N1 and H5N1 influenza viruses exhibit significantly high numbers of macrophages and neutrophils in the lungs compared to mice infected with low pathogenic (LP) viruses. Mice infected with the 1918 pandemic virus and a recent H5N1 human isolate show considerable similarities in overall lung cellularity, lung immune cell sub-population composition, and cellular immune temporal dynamics. Interestingly, while these similarities were observed, the HP H5N1 virus consistently elicited significantly higher levels of pro-inflammatory cytokines in whole lungs and primary human macrophages, revealing a potentially critical difference in the pathogenesis of H5N1 infections. Primary mouse and human macrophages and dendritic cells were also susceptible to 1918 and H5N1 influenza virus infection in vitro. These results together indicate that infection with HP influenza viruses such as H5N1 and the 1918 pandemic virus leads to a rapid cell recruitment of macrophages and neutrophils into the lungs, suggesting that these cells play a role in acute lung inflammation associated with HP influenza virus infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Glezen WP. Emerging infections: pandemic influenza. Epidemiol Rev. 1996;18:64–76. - PubMed
-
- Winternitz MC, Wason IM, McNamara FP. New Haven: Yale University Press; 1920. The Pathology of Influenza.
-
- LeCount ER. The Pathologic Anatomy of Influenzal Bronchopneumonia. JAMA. 1919;650–652
-
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

