Ultrafast time-resolved carotenoid to-bacteriochlorophyll energy transfer in LH2 complexes from photosynthetic bacteria
- PMID: 18671366
- PMCID: PMC3628606
- DOI: 10.1021/jp711946w
Ultrafast time-resolved carotenoid to-bacteriochlorophyll energy transfer in LH2 complexes from photosynthetic bacteria
Abstract
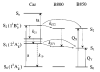
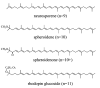
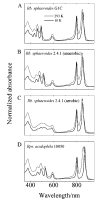
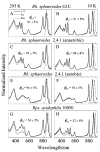
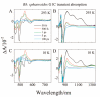
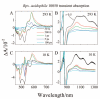
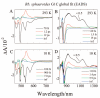
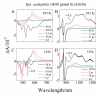
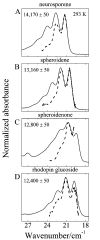
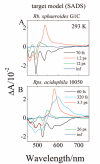
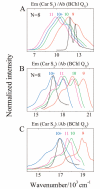
Steady-state and ultrafast time-resolved optical spectroscopic investigations have been carried out at 293 and 10 K on LH2 pigment-protein complexes isolated from three different strains of photosynthetic bacteria: Rhodobacter (Rb.) sphaeroides G1C, Rb. sphaeroides 2.4.1 (anaerobically and aerobically grown), and Rps. acidophila 10050. The LH2 complexes obtained from these strains contain the carotenoids, neurosporene, spheroidene, spheroidenone, and rhodopin glucoside, respectively. These molecules have a systematically increasing number of pi-electron conjugated carbon-carbon double bonds. Steady-state absorption and fluorescence excitation experiments have revealed that the total efficiency of energy transfer from the carotenoids to bacteriochlorophyll is independent of temperature and nearly constant at approximately 90% for the LH2 complexes containing neurosporene, spheroidene, spheroidenone, but drops to approximately 53% for the complex containing rhodopin glucoside. Ultrafast transient absorption spectra in the near-infrared (NIR) region of the purified carotenoids in solution have revealed the energies of the S1 (2(1)Ag-)-->S2 (1(1)Bu+) excited-state transitions which, when subtracted from the energies of the S0 (1(1)Ag-)-->S2 (1(1)Bu+) transitions determined by steady-state absorption measurements, give precise values for the positions of the S1 (2(1)Ag-) states of the carotenoids. Global fitting of the ultrafast spectral and temporal data sets have revealed the dynamics of the pathways of de-excitation of the carotenoid excited states. The pathways include energy transfer to bacteriochlorophyll, population of the so-called S* state of the carotenoids, and formation of carotenoid radical cations (Car*+). The investigation has found that excitation energy transfer to bacteriochlorophyll is partitioned through the S1 (1(1)Ag-), S2 (1(1)Bu+), and S* states of the different carotenoids to varying degrees. This is understood through a consideration of the energies of the states and the spectral profiles of the molecules. A significant finding is that, due to the low S1 (2(1)Ag-) energy of rhodopin glucoside, energy transfer from this state to the bacteriochlorophylls is significantly less probable compared to the other complexes. This work resolves a long-standing question regarding the cause of the precipitous drop in energy transfer efficiency when the extent of pi-electron conjugation of the carotenoid is extended from ten to eleven conjugated carbon-carbon double bonds in LH2 complexes from purple photosynthetic bacteria.
Figures

Similar articles
-
Role of B800 in carotenoid-bacteriochlorophyll energy and electron transfer in LH2 complexes from the purple bacterium Rhodobacter sphaeroides.J Phys Chem B. 2007 Jun 28;111(25):7422-31. doi: 10.1021/jp071395c. Epub 2007 Jun 5. J Phys Chem B. 2007. PMID: 17547450
-
Carotenoid radical cation formation in LH2 of purple bacteria: a quantum chemical study.J Phys Chem B. 2006 Nov 30;110(47):24200-6. doi: 10.1021/jp064568r. J Phys Chem B. 2006. PMID: 17125392
-
Dynamics of energy transfer from lycopene to bacteriochlorophyll in genetically-modified LH2 complexes of Rhodobacter sphaeroides.Biochemistry. 2002 Mar 26;41(12):4127-36. doi: 10.1021/bi011741v. Biochemistry. 2002. PMID: 11900556
-
The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes.Q Rev Biophys. 2006 Aug;39(3):227-324. doi: 10.1017/S0033583506004434. Epub 2006 Oct 12. Q Rev Biophys. 2006. PMID: 17038210 Review.
-
Intramolecular charge transfer and the function of vibronic excitons in photosynthetic light harvesting.Photosynth Res. 2024 Dec;162(2-3):139-156. doi: 10.1007/s11120-024-01095-5. Epub 2024 Apr 24. Photosynth Res. 2024. PMID: 38656684 Review.
Cited by
-
Reconstitution of Gloeobacter violaceus rhodopsin with a light-harvesting carotenoid antenna.Biochemistry. 2009 Nov 24;48(46):10948-55. doi: 10.1021/bi901552x. Biochemistry. 2009. PMID: 19842712 Free PMC article.
-
Ultrafast time-resolved spectroscopy of the light-harvesting complex 2 (LH2) from the photosynthetic bacterium Thermochromatium tepidum.Photosynth Res. 2011 Oct;110(1):49-60. doi: 10.1007/s11120-011-9692-7. Epub 2011 Oct 8. Photosynth Res. 2011. PMID: 21984346
-
Assembly of functional photosystem complexes in Rhodobacter sphaeroides incorporating carotenoids from the spirilloxanthin pathway.Biochim Biophys Acta. 2015 Feb;1847(2):189-201. doi: 10.1016/j.bbabio.2014.10.004. Epub 2014 Oct 27. Biochim Biophys Acta. 2015. PMID: 25449968 Free PMC article.
-
The Influence of the Number of Conjugated Double Bonds in Carotenoid Molecules on the Energy Transfer Efficiency to Bacteriochlorophyll in Light-Harvesting Complexes LH2 from Allochromatium vinosum Strain MSU.Dokl Biochem Biophys. 2018 Nov;483(1):321-325. doi: 10.1134/S160767291806008X. Epub 2019 Jan 3. Dokl Biochem Biophys. 2018. PMID: 30607730
-
Triplet excited state spectra and dynamics of carotenoids from the thermophilic purple photosynthetic bacterium Thermochromatium tepidum.Photosynth Res. 2011 Feb;107(2):177-86. doi: 10.1007/s11120-011-9620-x. Epub 2011 Jan 13. Photosynth Res. 2011. PMID: 21229315
References
-
- Angerhofer A, Bornhauser F, Gall A, Cogdell RJ. Chem. Phys. 1995;194:259.
-
- Frank HA, Cogdell RJ. Photochem. Photobiol. 1996;63:257. - PubMed
-
- Akahane J, Rondonuwu FS, Fiedor L, Watanabe Y, Koyama Y. Chem. Phys. Lett. 2004;393:184.
-
- Cogdell RJ. Pure Appl. Chem. 1985;57:723.
-
- McDermott G, Prince SM, Freer AA, Hawthornthwaite-Lawless AM, Papiz MZ, Cogdell RJ, Isaacs NW. Nature (London) 1995;374:517.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

