Water-soluble 2-hydroxyisophthalamides for sensitization of lanthanide luminescence
- PMID: 18671388
- PMCID: PMC3176712
- DOI: 10.1021/ic800328g
Water-soluble 2-hydroxyisophthalamides for sensitization of lanthanide luminescence
Abstract
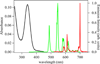
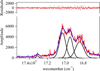
A series of octadentate ligands featuring the 2-hydroxyisophthalamide (IAM) antenna chromophore to sensitize Tb(III) and Eu(III) luminescence has been prepared and characterized. The length of the alkyl amine scaffold that links the four IAM moieties has been varied to investigate the effect of the ligand backbone on the stability and photophysical properties of the Ln(III) complexes. The amine backbones utilized in this study are N,N,N',N'-tetrakis-(2-aminoethyl)-ethane-1,2-diamine [H(2,2)-], N,N,N',N'-tetrakis-(2-aminoethyl)-propane-1,3-diamine [H(3,2)-], and N,N,N',N'-tetrakis-(2-aminoethyl)-butane-1,4-diamine [H(4,2)-]. These ligands also incorporate methoxyethylene [MOE] groups on each of the IAM chromophores to increase their water solubility. The aqueous ligand protonation constants and Tb(III) and Eu(III) formation constants were determined from solution thermodynamic studies. The resulting values indicate that at physiological pH the Eu(III) and Tb(III) complexes of H(2,2)-IAM-MOE and H(4,2)-IAM-MOE are sufficiently stable to prevent dissociation at nanomolar concentrations. The photophysical measurements for the Tb(III) complexes gave overall quantum yield values of 0.56, 0.39, and 0.52 respectively for the complexes with H(2,2)-IAM-MOE, H(3,2)-IAM-MOE, and H(4,2)-IAM-MOE, while the corresponding Eu(III) complexes displayed significantly weaker luminescence, with quantum yield values of 0.0014, 0.0015, and 0.0058, respectively. Analysis of the steady state Eu(III) emission spectra provides insight into the solution symmetries of the complexes. The combined solubility, stability, and photophysical performance of the Tb(III) complexes in particular make them well suited to serve as the luminescent reporter group in high sensitivity time-resolved fluoroimmunoassays.
Figures



Similar articles
-
Octadentate cages of Tb(III) 2-hydroxyisophthalamides: a new standard for luminescent lanthanide labels.J Am Chem Soc. 2011 Dec 14;133(49):19900-10. doi: 10.1021/ja2079898. Epub 2011 Nov 14. J Am Chem Soc. 2011. PMID: 22010878 Free PMC article.
-
From antenna to assay: lessons learned in lanthanide luminescence.Acc Chem Res. 2009 Apr 21;42(4):542-52. doi: 10.1021/ar800211j. Acc Chem Res. 2009. PMID: 19323456 Free PMC article.
-
Siderophore inspired tetra- and octadentate antenna ligands for luminescent Eu(III) and Tb(III) complexes.J Inorg Biochem. 2016 Sep;162:263-273. doi: 10.1016/j.jinorgbio.2016.01.006. Epub 2016 Jan 9. J Inorg Biochem. 2016. PMID: 26832605
-
Circularly polarized luminescence in enantiopure europium and terbium complexes with modular, all-oxygen donor ligands.Inorg Chem. 2009 Sep 7;48(17):8469-79. doi: 10.1021/ic901079s. Inorg Chem. 2009. PMID: 19639983 Free PMC article.
-
Quantifying the formation of chiral luminescent lanthanide assemblies in an aqueous medium through chiroptical spectroscopy and generation of luminescent hydrogels.Faraday Discuss. 2015;185:413-31. doi: 10.1039/c5fd00105f. Faraday Discuss. 2015. PMID: 26404059
Cited by
-
Evaluation of macrocyclic hydroxyisophthalamide ligands as chelators for zirconium-89.PLoS One. 2017 Jun 2;12(6):e0178767. doi: 10.1371/journal.pone.0178767. eCollection 2017. PLoS One. 2017. PMID: 28575044 Free PMC article.
-
1-Methyl-3-hydroxy-pyridin-2-one complexes of near infra-red emitting lanthanides: efficient sensitization of Yb(III) and Nd(III) in aqueous solution.Inorg Chem. 2010 May 3;49(9):4156-66. doi: 10.1021/ic902219t. Inorg Chem. 2010. PMID: 20364838 Free PMC article.
-
A single sensitizer for the excitation of visible and NIR lanthanide emitters in water with high quantum yields.Angew Chem Int Ed Engl. 2012 Mar 5;51(10):2371-4. doi: 10.1002/anie.201106748. Epub 2012 Jan 24. Angew Chem Int Ed Engl. 2012. PMID: 22271666 Free PMC article.
-
Strong Circularly Polarized Luminescence from Highly Emissive Terbium Complexes in Aqueous Solution.Eur J Inorg Chem. 2010 Jul 1;2010(21):3343-3347. doi: 10.1002/ejic.201000309. Eur J Inorg Chem. 2010. PMID: 20730030 Free PMC article.
-
Octadentate cages of Tb(III) 2-hydroxyisophthalamides: a new standard for luminescent lanthanide labels.J Am Chem Soc. 2011 Dec 14;133(49):19900-10. doi: 10.1021/ja2079898. Epub 2011 Nov 14. J Am Chem Soc. 2011. PMID: 22010878 Free PMC article.
References
-
- Bünzli J-CG. In: Lanthanide Probes in Life, Chemical and Earth Sciences: Theory and Practice. Bünzli J-CG, Choppin GR, editors. Amsterdam: Elsevier; 1989.
-
- Hemmila I, Laitala V. J. Fluor. 2005;15:529–542. - PubMed
-
- Charbonniere LJ, Hildebrandt N, Ziessel RF, Loehmannsroeben H-G. J. Am. Chem. Soc. 2006;39:12800–12809. - PubMed
-
- Hemmila IJ. Alloys Compd. 1995;225:480–485.
-
- Bünzli J-C, Piguet C. Chem. Soc. Rev. 2005;34:1048–1077. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

