Cell-based therapy in ischemic stroke
- PMID: 18671663
- PMCID: PMC2753686
- DOI: 10.1586/14737175.8.8.1193
Cell-based therapy in ischemic stroke
Abstract
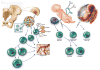
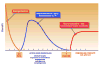
Cell-based therapy for stroke represents a third wave of therapeutics for stroke and one focused on restorative processes with a longer time window of opportunity than neuroprotective therapies. An early time window, within the first week after stroke, is an opportunity for intravenously delivered bone marrow and perinatally derived cells that can home to areas of tissue injury and target brain remodeling. Allogeneic cells will likely be the most scalable and commercially viable product. Later time windows, months after stroke, may be opportunities for intracerebral transplantation of neuronally differentiated cell types. An integrated approach of cell-based therapy with early-phase clinical trials and continued preclinical work with focus on mechanisms of action is needed.
Figures
References
-
- Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. - PubMed
-
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nat Med. 2008 - PubMed
-
- Nadal-Ginard B, Fuster V. Myocardial cell therapy at the crossroads. Nat Clin Pract Cardiovasc Med. 2007;4(1):1. - PubMed
-
* An editorial calling for a moratorium on cell-based therapy clinical trials.
-
- Cardiac cell therapy: bench or bedside? Nat Clin Pract Cardiovasc Med. 2007;4(8):403. - PubMed
-
* A counter argument to the editorial calling for a moratorium on cell-based therapy in cardiovascular diseases.
-
- Bath PM, Sprigg N. Colony stimulating factors (including erythropoietin, granulocyte colony stimulating factor and analogues) for stroke. Cochrane Database Syst Rev. 2006;3:CD005207. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
