The roles of CD8 central and effector memory T-cell subsets in allograft rejection
- PMID: 18671680
- PMCID: PMC4872301
- DOI: 10.1111/j.1600-6143.2008.02335.x
The roles of CD8 central and effector memory T-cell subsets in allograft rejection
Abstract
The contribution of secondary lymphoid tissue-homing central memory T cells (T(CM)) and peripheral tissue-homing effector memory T cells (T(EM)) to allograft rejection is not known. We tested whether T(EM) is the principal subset responsible for allograft rejection due to the nonlymphoid location of target antigens. Skin allograft rejection was studied after transferring either CD8 T(CM) or T(EM) to wild-type mice and to mice that lack secondary lymphoid tissues. We found that CD8 T(CM) and T(EM) were equally effective at rejecting allografts in wild-type hosts. However, CD8 T(EM) were significantly better than T(CM) at rejecting allografts in the absence of secondary lymphoid tissues. CD8 T(CM) were dependent upon secondary lymphoid tissues more than T(EM) for optimal differentiation into effectors that migrate into the allograft. Recall of either CD8 T(CM) or T(EM) led to accumulation of T(EM) after allograft rejection. These findings indicate that either CD8 T(CM) or T(EM) mediate allograft rejection but T(EM) have an advantage over T(CM) in immune surveillance of peripheral tissues, including transplanted organs.
Figures


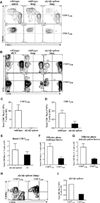
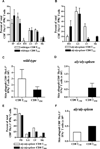

Similar articles
-
NK cells delay allograft rejection in lymphopenic hosts by downregulating the homeostatic proliferation of CD8+ T cells.J Immunol. 2010 Jun 15;184(12):6649-57. doi: 10.4049/jimmunol.0903729. Epub 2010 May 14. J Immunol. 2010. PMID: 20483732
-
Alloreactive CD8 T-cell primed/memory responses and accelerated graft rejection in B-cell-deficient sensitized mice.Transplantation. 2011 May 27;91(10):1075-81. doi: 10.1097/TP.0b013e31821578da. Transplantation. 2011. PMID: 21427633 Free PMC article.
-
Type I interferons are not critical for skin allograft rejection or the generation of donor-specific CD8+ memory T cells.Am J Transplant. 2010 Jan;10(1):162-7. doi: 10.1111/j.1600-6143.2009.02871.x. Epub 2009 Nov 24. Am J Transplant. 2010. PMID: 19951284 Free PMC article.
-
Memory CD8+ T cell responses to cancer.Semin Immunol. 2020 Jun;49:101435. doi: 10.1016/j.smim.2020.101435. Epub 2020 Nov 30. Semin Immunol. 2020. PMID: 33272898 Free PMC article. Review.
-
Role of secondary lymphoid tissues in primary and memory T-cell responses to a transplanted organ.Transplant Rev (Orlando). 2010 Jan;24(1):32-41. doi: 10.1016/j.trre.2009.09.003. Epub 2009 Oct 20. Transplant Rev (Orlando). 2010. PMID: 19846289 Free PMC article. Review.
Cited by
-
Heterogeneity within T Cell Memory: Implications for Transplant Tolerance.Front Immunol. 2012 Mar 1;3:36. doi: 10.3389/fimmu.2012.00036. eCollection 2012. Front Immunol. 2012. PMID: 22566919 Free PMC article.
-
Analysis of T-Cell Receptor Repertoire in Transplantation: Fingerprint of T Cell-mediated Alloresponse.Front Immunol. 2022 Jan 12;12:778559. doi: 10.3389/fimmu.2021.778559. eCollection 2021. Front Immunol. 2022. PMID: 35095851 Free PMC article. Review.
-
B cells help alloreactive T cells differentiate into memory T cells.Am J Transplant. 2010 Sep;10(9):1970-80. doi: 10.1111/j.1600-6143.2010.03223.x. Am J Transplant. 2010. PMID: 20883532 Free PMC article.
-
Interferon gamma licensing of human dendritic cells in T-helper-independent CD8+ alloimmunity.Blood. 2010 Oct 21;116(16):3089-98. doi: 10.1182/blood-2010-02-268623. Epub 2010 Jul 19. Blood. 2010. PMID: 20644110 Free PMC article.
-
Coupled regulation of interleukin-12 receptor beta-1 of CD8+ central memory and CCR7-negative memory T cells in an early alloimmunity in liver transplant recipients.Clin Exp Immunol. 2010 Jun;160(3):420-30. doi: 10.1111/j.1365-2249.2010.04117.x. Epub 2010 Mar 16. Clin Exp Immunol. 2010. PMID: 20345976 Free PMC article.
References
-
- Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-γ-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. - PubMed
-
- Najafian N, Salama A, Fedoseyeva E, Benichou G, Sayegh M. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol. 2002;13:252–259. - PubMed
-
- Pearl JP, Parris J, Hale DA, Hoffman SC, Bernstein WB, McCoy KL, et al. Immunocompetent T cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. - PubMed
-
- Valujskikh A, Pantenburg B, Heeger P. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

